Improvement of solubility and dissolution properties of clotrimazole by solid dispersions and inclusion complexes
- PMID: 22923864
- PMCID: PMC3425063
- DOI: 10.4103/0250-474X.98995
Improvement of solubility and dissolution properties of clotrimazole by solid dispersions and inclusion complexes
Abstract
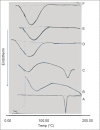
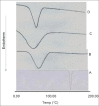
Solid dispersions of a slightly water-soluble drug, clotrimazole, were prepared in different weight ratios using polyethyleneglycol 4000 and different molecular weight polyvinyl pyrrolidones as carriers. Moreover, binary and ternary β-cyclodextrin complexes were prepared in different molar ratios. Both solid dispersions and β-cyclodextrin complexes were prepared by solvent evaporation technique. A phase solubility method was used to evaluate the effect of the tested carriers on the aqueous solubility of clotrimazole. The dissolution of all the preparations was tested using the USP paddle method. The selected solid dispersions and inclusion complexes were characterized by differential scanning calorimetry and X-ray powder diffractometry studies, and results clarified the role of the tested carriers in decreasing the crystallinity of clotrimazole and complexing abilities. Based on physical characters and in vitro drug release pattern, polyvinylpyrrolidone solid dispersions (1:1 weight ratio) and ternary cyclodextrin complexes (clotrimazole-β-cyclodextrin complexes with either polymer, 1:1 molar ratio) were selected as ideal batches for suppositories. Suppocire AM/50 mg carbopol 940, was chosen as a suppository base and the suppositories were prepared by molding technique. The prepared suppositories were characterized for weight variation, softening time and drug content. All these properties were found to be ideal. The in vitro drug release pattern was determined in citrate buffer (pH 4.5) containing 1% sodium lauryl sulfate. The in vitro release of clotrimazole from its solid dispersions and inclusion complexes incorporated suppositories was markedly improved when compared to the intact drug incorporated suppositories. Polyvinyl pyrrolidone solid dispersions incorporated suppositories were found to possess excellent antifungal activity.
Keywords: Clotrimazole; polyethyleneglycol; polyvinylpyrrolidone; suppository; β-cyclodextrin.
Figures




References
-
- Ross RA, Lee MT, Onderdonk AB. Effect of Candida albicans infection and clotrimazole treatment on vaginal microflora in vitro. Obstet Gynecol. 1995;86:925–30. - PubMed
-
- Realdon N, Ragazzi E, Ragazzi E. Effect of drug solubility on in vitro availability rate from suppositories with polyethylene glycol excipients. Pharmazie. 2001;56:163–7. - PubMed
-
- Gowthamarajan K, Girirajkulkarni T, Venkateswaran G, Samanta MK, Suresh B. Formulation and dissolution properties of meloxicam solid dispersion incorporated suppositories. Indian J Pharm Sci. 2002;64:525–8.
-
- Samy EM, Hassan MA, Tous SS, Rhodes CT. Improvement of availability of allopurinol from pharmaceutical dosage forms I – suppositories. Eur J Pharm Biopharm. 2000;49:119–27. - PubMed
-
- Usayapant A, Iyer BR. The effect of 2-hydropropyl-betacyclodextrin on in vitro drug release of steroids from suppositories. Drug Develop Ind Pharm. 1999;25:390–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
