Processing pathway dependence of amorphous silica nanoparticle toxicity: colloidal vs pyrolytic
- PMID: 22924492
- PMCID: PMC3505689
- DOI: 10.1021/ja304907c
Processing pathway dependence of amorphous silica nanoparticle toxicity: colloidal vs pyrolytic
Abstract
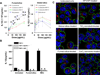
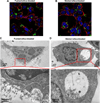
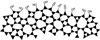
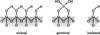
We have developed structure/toxicity relationships for amorphous silica nanoparticles (NPs) synthesized through low-temperature colloidal (e.g., Stöber silica) or high-temperature pyrolysis (e.g., fumed silica) routes. Through combined spectroscopic and physical analyses, we have determined the state of aggregation, hydroxyl concentration, relative proportion of strained and unstrained siloxane rings, and potential to generate hydroxyl radicals for Stöber and fumed silica NPs with comparable primary particle sizes (16 nm in diameter). On the basis of erythrocyte hemolytic assays and assessment of the viability and ATP levels in epithelial and macrophage cells, we discovered for fumed silica an important toxicity relationship to postsynthesis thermal annealing or environmental exposure, whereas colloidal silicas were essentially nontoxic under identical treatment conditions. Specifically, we find for fumed silica a positive correlation of toxicity with hydroxyl concentration and its potential to generate reactive oxygen species (ROS) and cause red blood cell hemolysis. We propose fumed silica toxicity stems from its intrinsic population of strained three-membered rings (3MRs) along with its chainlike aggregation and hydroxyl content. Hydrogen-bonding and electrostatic interactions of the silanol surfaces of fumed silica aggregates with the extracellular plasma membrane cause membrane perturbations sensed by the Nalp3 inflammasome, whose subsequent activation leads to secretion of the cytokine IL-1β. Hydroxyl radicals generated by the strained 3MRs in fumed silica, but largely absent in colloidal silicas, may contribute to the inflammasome activation. Formation of colloidal silica into aggregates mimicking those of fumed silica had no effect on cell viability or hemolysis. This study emphasizes that not all amorphous silicas are created equal and that the unusual toxicity of fumed silica compared to that of colloidal silica derives from its framework and surface chemistry along with its fused chainlike morphology established by high-temperature synthesis (>1300 °C) and rapid thermal quenching.
Figures





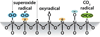
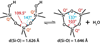
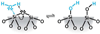

References
-
- Lutgens FK, Tarbuck EJ. Essentials of Geology. 7th ed. Prentice Hall; 2000.
-
- Brinkmann U, Ettlinger M, Kerner D, Schmoll R. In: Colloidal Silica: Fundamentals and Applications. Bergna HE, Roberts WO, editors. Hoboken, NJ: CRC Press; 2005. pp. 575–588.
-
- Pratsinis S. Progress in Energy and Combustion Science. 1998;24:197–219.
-
- Brinker CJ, Scherer GW. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. San Diego, CA: Academic Press; 1990.
-
- Iler RK. The Chemistry of Silica. New York: John Wiley and Sons; 1979.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

