NF-κB-dependent IL-8 induction by prostaglandin E(2) receptors EP(1) and EP(4)
- PMID: 22924768
- PMCID: PMC3579289
- DOI: 10.1111/j.1476-5381.2012.02182.x
NF-κB-dependent IL-8 induction by prostaglandin E(2) receptors EP(1) and EP(4)
Abstract
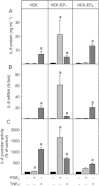
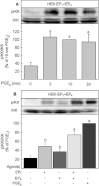
Background and purpose: Recent studies suggested a role for PGE(2) in the expression of the chemokine IL-8. PGE(2) signals via four different GPCRs, EP(1) -EP(4) . The role of EP(1) and EP(4) receptors for IL-8 induction was studied in HEK293 cells, overexpressing EP(1) (HEK-EP(1) ), EP(4) (HEK-EP(4) ) or both receptors (HEK-EP(1) + EP(4) ).
Experimental approach: IL-8 mRNA and protein induction and IL-8 promoter and NF-κB activation were assessed in EP expressing HEK cells.
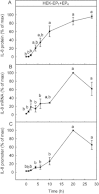
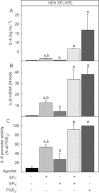
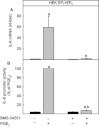
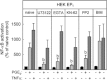
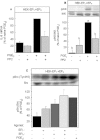
Key results: In HEK-EP(1) and HEK-EP(1) + EP(4) but not HEK or HEK-EP(4) cells, PGE(2) activated the IL-8 promoter and induced IL-8 mRNA and protein synthesis. Stimulation of HEK-EP(1) + EP(4) cells with an EP(1) -specific agonist activated IL-8 promoter and induced IL-8 mRNA and protein, whereas a specific EP(4) agonist neither activated the IL-8 promoter nor induced IL-8 mRNA and protein synthesis. Simultaneous stimulation of HEK- EP(1) + EP(4) cells with both agonists activated IL-8 promoter and induced IL-8 mRNA to the same extent as PGE(2) . In HEK-EP(1) + EP(4) cells, PGE(2) -mediated IL-8 promoter activation and IL-8 mRNA induction were blunted by inhibition of IκB kinase. PGE(2) activated NF-κB in HEK-EP(1) , HEK-EP(4) and HEK-EP(1) + EP(4) cells. In HEK-EP(1) + EP(4) cells, simultaneous activation of both receptors was needed for maximal PGE(2) -induced NF-κB activation. PGE(2) -stimulated NF-κB activation by EP(1) was blocked by inhibitors of PLC, calcium-signalling and Src-kinase, whereas that induced by EP(4) was only blunted by Src-kinase inhibition.
Conclusions and implications: These findings suggest that PGE(2) -mediated NF-κB activation by simultaneous stimulation of EP(1) and EP(4) receptors induces maximal IL-8 promoter activation and IL-8 mRNA and protein induction.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures



References
-
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. - PubMed
-
- Caristi S, Piraino G, Cucinotta M, Valenti A, Loddo S, Teti D. Prostaglandin E2 induces interleukin-8 gene transcription by activating C/EBP homologous protein in human T lymphocytes. J Biol Chem. 2005;280:14433–14442. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

