Preventing relapse in recurrent depression using mindfulness-based cognitive therapy, antidepressant medication or the combination: trial design and protocol of the MOMENT study
- PMID: 22925198
- PMCID: PMC3469366
- DOI: 10.1186/1471-244X-12-125
Preventing relapse in recurrent depression using mindfulness-based cognitive therapy, antidepressant medication or the combination: trial design and protocol of the MOMENT study
Abstract
Background: Depression is a common psychiatric disorder characterized by a high rate of relapse and recurrence. The most commonly used strategy to prevent relapse/recurrence is maintenance treatment with antidepressant medication (mADM). Recently, it has been shown that Mindfulness-Based Cognitive Therapy (MBCT) is at least as effective as mADM in reducing the relapse/recurrence risk. However, it is not yet known whether combination treatment of MBCT and mADM is more effective than either of these treatments alone. Given the fact that most patients have a preference for either mADM or for MBCT, the aim of the present study is to answer the following questions. First, what is the effectiveness of MBCT in addition to mADM? Second, how large is the risk of relapse/recurrence in patients withdrawing from mADM after participating in MBCT, compared to those who continue to use mADM after MBCT?
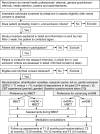
Methods/design: Two parallel-group, multi-center randomized controlled trials are conducted. Adult patients with a history of depression (3 or more episodes), currently either in full or partial remission and currently treated with mADM (6 months or longer) are recruited. In the first trial, we compare mADM on its own with mADM plus MBCT. In the second trial, we compare MBCT on its own, including tapering of mADM, with mADM plus MBCT. Follow-up assessments are administered at 3-month intervals for 15 months. Primary outcome is relapse/recurrence. Secondary outcomes are time to, duration and severity of relapse/recurrence, quality of life, personality, several process variables, and incremental cost-effectiveness ratio.
Discussion: Taking into account patient preferences, this study will provide information about a) the clinical and cost-effectiveness of mADM only compared with mADM plus MBCT, in patients with a preference for mADM, and b) the clinical and cost-effectiveness of withdrawing from mADM after MBCT, compared with mADM plus MBCT, in patients with a preference for MBCT.
Trial registration: ClinicalTrials.gov: NCT00928980.
Figures
Similar articles
-
Adding mindfulness-based cognitive therapy to maintenance antidepressant medication for prevention of relapse/recurrence in major depressive disorder: Randomised controlled trial.J Affect Disord. 2015 Nov 15;187:54-61. doi: 10.1016/j.jad.2015.08.023. Epub 2015 Aug 18. J Affect Disord. 2015. PMID: 26318271 Clinical Trial.
-
Patients with a preference for medication do equally well in mindfulness-based cognitive therapy for recurrent depression as those preferring mindfulness.J Affect Disord. 2016 May;195:32-9. doi: 10.1016/j.jad.2016.01.041. Epub 2016 Jan 28. J Affect Disord. 2016. PMID: 26852095 Clinical Trial.
-
The effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse/recurrence: results of a randomised controlled trial (the PREVENT study).Health Technol Assess. 2015 Sep;19(73):1-124. doi: 10.3310/hta19730. Health Technol Assess. 2015. PMID: 26379122 Free PMC article. Clinical Trial.
-
Approaches for discontinuation versus continuation of long-term antidepressant use for depressive and anxiety disorders in adults.Cochrane Database Syst Rev. 2021 Apr 15;4(4):CD013495. doi: 10.1002/14651858.CD013495.pub2. Cochrane Database Syst Rev. 2021. PMID: 33886130 Free PMC article.
-
Comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder in adults.Cochrane Database Syst Rev. 2019 May 20;5(5):CD012855. doi: 10.1002/14651858.CD012855.pub2. Cochrane Database Syst Rev. 2019. PMID: 31106850 Free PMC article.
Cited by
-
Discontinuing antidepressant medication after mindfulness-based cognitive therapy: a mixed-methods study exploring predictors and outcomes of different discontinuation trajectories, and its facilitators and barriers.BMJ Open. 2020 Nov 11;10(11):e039053. doi: 10.1136/bmjopen-2020-039053. BMJ Open. 2020. PMID: 33177138 Free PMC article.
-
Teacher Competence in Mindfulness-Based Cognitive Therapy for Depression and Its Relation to Treatment Outcome.Mindfulness (N Y). 2017;8(4):960-972. doi: 10.1007/s12671-016-0672-z. Epub 2017 Jan 12. Mindfulness (N Y). 2017. PMID: 28757901 Free PMC article.
-
Molecular serum signature of treatment resistant depression.Psychopharmacology (Berl). 2016 Aug;233(15-16):3051-9. doi: 10.1007/s00213-016-4348-0. Epub 2016 Jun 20. Psychopharmacology (Berl). 2016. PMID: 27325393
-
Managing Antidepressant Discontinuation: A Systematic Review.Ann Fam Med. 2019 Jan;17(1):52-60. doi: 10.1370/afm.2336. Ann Fam Med. 2019. PMID: 30670397 Free PMC article.
-
Perceptions, Uses of, and Interests in Complementary Health Care Approaches in Depressed Pregnant Women: The PAW Survey.J Evid Based Complementary Altern Med. 2017 Jan;22(1):81-95. doi: 10.1177/2156587216641829. Epub 2016 Apr 12. J Evid Based Complementary Altern Med. 2017. PMID: 27071640 Free PMC article.
References
-
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):85–855. - PubMed
-
- Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, Warshaw M, Maser JD. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000–1006. - PubMed
-
- National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. London: National Institute for Health and Clinical Excellence; 2004. NICE guidance.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical