Standardization of a method to detect bovine sperm-bound antisperm antibodies by flow cytometry
- PMID: 22925638
- PMCID: PMC3444527
- DOI: 10.1016/j.theriogenology.2012.06.026
Standardization of a method to detect bovine sperm-bound antisperm antibodies by flow cytometry
Abstract
The objectives were to standardize some methodological and analytical aspects of a direct technique to detect sperm-bound antisperm antibodies (ASAs) in bovine semen using flow cytometry, including the effects of prefixation of sperm membranes with formalin buffer solution and inclusion of dead cells in the analysis. Fourteen Angus bulls, including ASA-positive (experimentally induced ASAs) and 10 reproductively normal ASA-negative bulls, were used. Fixation of sperm membranes had no significant effect on the percentage of IgG- or IgA-bound spermatozoa detected by flow cytometry. However, including dead cells in the analysis increased the percentage of IgG-bound spermatozoa in fixed (live and dead 18.6 ± 9.7% and live 1.3 ± 0.5%; median ± SEM) and nonfixed samples (live and dead 18.8 ± 9.2%, live 1.5 ± 0.6%; P = 0.0029), as well as IgA-bound spermatozoa in fixed (live and dead 16.3 ± 6.4%, live 0.3 ± 0.5%) and nonfixed samples (live and dead 21.4 ± 4.6%, live 1.0 ± 0.5%; P = 0.0041) in semen from ASA-negative bulls. Intrasample, intra-assay, and interassay coefficients of variation (CV) were 0.8%, 4.6%, and 5.3%, respectively, for determination of sperm-bound IgG, and were 2.8%, 8.4%, and 40.3% for determination of sperm-bound IgA. Despite the high interassay CV for IgA determination, all ASA-positive bulls consistently had high percentages of IgA-bound spermatozoa. Flow cytometry correctly identified ASA-positive bulls. Confocal laser microscopy confirmed binding of ASAs to sperm heads and cytoplasmic droplets, and less frequently to midpieces and principal piece. In conclusion, although fixation was not necessary, dead cells should be excluded from the analysis, because ejaculates with a large proportion of dead cells can yield false-positive results. Flow cytometry was accurate and reliable for detection of sperm-bound IgG and IgA and discrimination between ASA-positive and ASA-negative bulls.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures

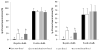

References
-
- Romrell LJ, O’Rand MG. Capping and ultrastructural localization of sperm surface isoantigens during spermatogenesis. Dev Biol. 1978;63:76–83. - PubMed
-
- Comhaire FH, Mahmound AMA, Depuydt CE, Zalata AA, Christophe AB. Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: the andrologist’s point of view. Human Reprod Update. 1999;5:939–98. - PubMed
-
- Zralý Z, Benová J, Šišák M, Diblíková I, Švecová D, Zajícová A, Vêžník Z. Occurrence of antibodies to sperms in blood sera of bulls and boars. Vet Med – Czech. 1998;43:197–204.
-
- Hegazi AG, Ezzo O. Serum auto antibodies in buffaloes and cattle naturally infected with IBRV and brucella. Buffalo J. 1995;3:325–30.
-
- Perez T, Carrasco LW. Autoimmunizacion espermatica en sementales bovinos como causa de subfertilidad. IVth Int Congr Anim Reprod Artif Insem. 1964;5:527.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

