The RanBP2/RanGAP1*SUMO1/Ubc9 complex: a multisubunit E3 ligase at the intersection of sumoylation and the RanGTPase cycle
- PMID: 22925898
- PMCID: PMC3474663
- DOI: 10.4161/nucl.21980
The RanBP2/RanGAP1*SUMO1/Ubc9 complex: a multisubunit E3 ligase at the intersection of sumoylation and the RanGTPase cycle
Abstract
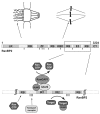
Posttranslational modification of proteins with SUMO and the RanGTP/GDP cycle are two essential cellular mechanisms contributing directly or indirectly to almost every cellular event. The SUMO E3 ligase RanBP2 (Nup358) and the Ran GTPase activating protein (RanGAP1) are known to form a stable complex throughout the cell cycle suggesting a link between sumoylation of proteins and RanGTP hydrolysis. In a recent study we demonstrated that the stable complex of RanBP2, sumoylated RanGAP1 and Ubc9 (and not RanBP2 by itself) represents the physiologically relevant form of the SUMO ligase. Characterization of the interactions reveals an intricate proximity of two catalytic activities, sumoylation and RanGTP hydrolysis. In this ExtraView we summarize our results and discuss some ideas about a potential coupling of both processes.
Figures
Comment on
- Werner A, Flotho A, Melchior F. The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase. Mol Cell. 2012;46:287–98. doi: 10.1016/j.molcel.2012.02.017. doi: 10.1016/j.molcel.2012.02.017
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
