Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies
- PMID: 22925968
- PMCID: PMC3502232
- DOI: 10.4161/mabs.21379
Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies
Abstract
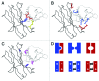
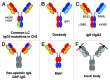
The development of bispecific antibodies has attracted substantial interest, and many different formats have been described. Those specifically containing an Fc part are mostly tetravalent, such as stabilized IgG-scFv fusions or dual-variable domain (DVD) IgGs. However, although they exhibit IgG-like properties and technical developability, these formats differ in size and geometry from classical IgG antibodies. Thus, considerable efforts focus on bispecific heterodimeric IgG antibodies that more closely mimic natural IgG molecules. The inherent chain association problem encountered when producing bispecific heterodimeric IgG antibodies can be overcome by several methods. While technologies like knobs-into-holes (KiH) combined with a common light chain or the CrossMab technology enforce the correct chain association, other approaches, e.g., the dual-acting Fab (DAF) IgGs, do not rely on a heterodimeric Fc part. This review discusses the state of the art in bispecific heterodimeric IgG antibodies, with an emphasis on recent progress.
Keywords: CrossMab; DAF; antibody; bispecific; heavy chain; heterodimerization; knobs-into-holes; light chain.
Figures
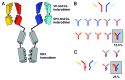

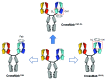
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
