Trauma patients' elevated tumor necrosis related apoptosis inducing ligand (TRAIL) contributes to increased T cell apoptosis
- PMID: 22926077
- PMCID: PMC3448438
- DOI: 10.1016/j.clim.2012.07.010
Trauma patients' elevated tumor necrosis related apoptosis inducing ligand (TRAIL) contributes to increased T cell apoptosis
Abstract
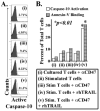
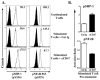
Immunosuppression resulting from excessive post-trauma apoptosis of hyperactivated T cells is controversial. TRAIL mediated T cell apoptosis decreases highly activated T cells' responses. Caspase-10, a particular TRAIL target, was increased in trauma patients' T cells with concomitantly elevated plasma TRAIL levels. These patients' T cells developed anergy, implicating increased TRAIL-mediated T cell apoptosis in post-trauma T cell anergy. Control T cells cultured with patients' sera containing high TRAIL levels increased their caspase-10 activity and apoptosis. Stimulated primary T cells are TRAIL apoptosis resistant. Increased plasma thrombospondin-1 and T cell expression of CD47, a thrombospondin-1 receptor, preceded patients' T cell anergy. CD47 triggering of T cells increased their sensitivity to TRAIL-induced apoptosis. Augmentation of T cell TRAIL-induced apoptosis was secondary to CD47 triggered activation of the Src homology-containing phosphatase-1 (SHP-1) and was partially blocked by a SHP-1 inhibitor. We suggest that combined post-trauma CD47 triggering, SHP-1 mediated NFκB suppression, and elevated TRAIL levels increase patients' CD47 expressing T cell apoptosis, thus contributing to subsequent T cell anergy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Wesche DE, Lomas-Neira JL, Perl M, Chung C-S, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. - PubMed
-
- Zhang Y, Liang L, Wu W, Gao Y, Chen ZB, Liang ZY, et al. Resuscitation with hydroxyethyl starch solution prevents CD4+ T-lymphocyte apoptosis and modulates the balance of T helper type 1 and T helper type 2 responses in the rat with traumatic virgule/shill hemorrhagic shock. Shock. 2008;30:692–698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

