Paladin is an antiphosphatase that regulates neural crest cell formation and migration
- PMID: 22926139
- PMCID: PMC3466348
- DOI: 10.1016/j.ydbio.2012.08.007
Paladin is an antiphosphatase that regulates neural crest cell formation and migration
Abstract
Although a network of transcription factors that specifies neural crest identity in the ectoderm has been defined, expression of neural crest transcription factors does not guarantee eventual migration as a neural crest cell. While much work has gone into determining regulatory relationships within the transcription factor network, the ability of protein modifications like phosphorylation to modulate the function of neural crest regulatory factors and determine when and where they are active also has crucial implications. Paladin, which was previously classified as a phosphatase based on sequence similarity, is expressed in chick neural crest precursors and is maintained throughout their epithelial to mesenchymal transition and migration. Loss of Paladin delays the expression of transcription factors Snail2 and Sox10 in premigratory neural crest cells, but does not affect accumulation of FoxD3, Cad6B or RhoB, indicating that Paladin differentially modulates the expression of genes previously thought to be coregulated within the neural crest gene regulatory network. Both gain and loss of Paladin function result in disrupted neural crest migration, reinforcing the importance of precisely regulated phosphorylation for neural crest migration. Mutation of critical, catalytic cysteine residues within Paladin's predicted phosphatase active site motifs did not abolish the function of Paladin in the neural crest. Collectively, these data indicate that Paladin is an antiphosphatase that modulates the activity of specific neural crest regulatory factors during neural crest development. Our work identifies a novel regulator of phosphorylation status that provides an additional layer of regulation in the neural crest.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

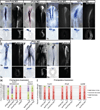
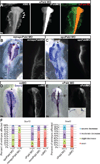



References
-
- Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. - PubMed
-
- Basch M, Bronner-Fraser M, Garcia-Castro M. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

