Cellular toxicity of mutant SOD1 protein is linked to an easily soluble, non-aggregated form in vitro
- PMID: 22926189
- PMCID: PMC3549044
- DOI: 10.1016/j.nbd.2012.08.010
Cellular toxicity of mutant SOD1 protein is linked to an easily soluble, non-aggregated form in vitro
Abstract
Mutations in superoxide dismutase 1 (SOD1) are found in approximately 20% of patients with familial amyotrophic lateral sclerosis. The propensity of mutant SOD1 to form aggregates in pathologically affected cells (i.e. motor neurons) has implicated these poorly soluble protein aggregates and/or their misfolded soluble precursors as being instrumental to the disease process. We investigated the relative solubility and toxicity of four different mutant SOD1 proteins in a cell-based model system and demonstrate that the mutant, misfolded SOD1 proteins that are the most soluble are also the most toxic. This toxicity was ameliorated by upregulating heat-shock protein chaperones in order to refold the soluble, misfolded protein, regardless of the presence of poorly soluble SOD1. We further demonstrate that increasing the solubility of a SOD1 mutant protein that is both poorly soluble and non-toxic, as compared to other mutant proteins, resulted in remarkably increased toxicity of the mutant SOD1. Again, this increased toxicity was attenuated by upregulating heat-shock protein chaperones in order to refold the soluble, misfolded proteins. These findings implicate easily soluble, misfolded SOD1 as being toxic to the cell and support the hypothesis that reducing solubility of mutant SOD1 proteins through aggregation may occur as a self-protective response in the cell.
Keywords: Aggregation; Amyotrophic lateral sclerosis; Misfold; SOD1; Solubility.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
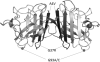
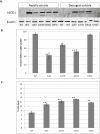

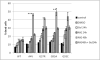



References
-
- Batulan Z, Taylor DM, Aarons RJ, Minotti S, Doroudchi MM, Nalbantoglu J, et al. Induction of multiple heat shock proteins and neuroprotection in a primary culture model of familial amyotrophic lateral sclerosis. Neurobiol Dis. 2006;24:213–225. - PubMed
-
- Beckmann RP, Mizzen LA, Welch WJ. Interaction of Hsp-70 with Newly Synthesized Proteins - Implications for Protein Folding and Assembly. Science. 1990;248:850–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

