Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: a review of the literature from 2000-2012
- PMID: 22926303
- PMCID: PMC6268307
- DOI: 10.3390/molecules170910192
Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: a review of the literature from 2000-2012
Abstract
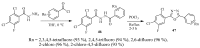
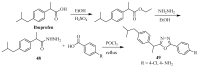
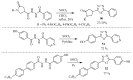
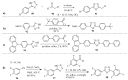
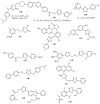
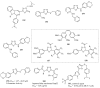
This review provides readers with an overview of the main synthetic methodologies for 1,3,4-oxadiazole derivatives, and of their broad spectrum of pharmacological activities as reported over the past twelve years.
Figures
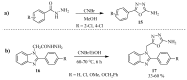
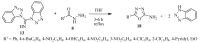
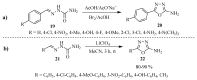
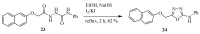

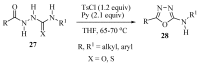
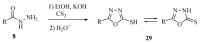
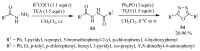
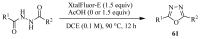
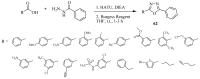
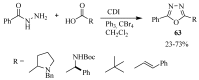
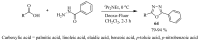

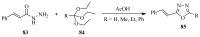
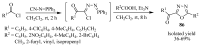
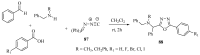
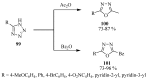
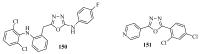
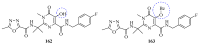
Similar articles
-
Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles.Eur J Med Chem. 2007 Feb;42(2):235-42. doi: 10.1016/j.ejmech.2006.10.003. Epub 2006 Nov 28. Eur J Med Chem. 2007. PMID: 17129641
-
Hypervalent iodine(III) mediated synthesis of novel unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles as antibacterial and antifungal agents.Eur J Med Chem. 2010 Sep;45(9):4252-7. doi: 10.1016/j.ejmech.2010.06.023. Epub 2010 Jun 22. Eur J Med Chem. 2010. PMID: 20630627
-
A novel synthesis and antimicrobial activity of 1-[(Substituted-phenyl) sulfonyl]pyrrolidin-2-ones.J Enzyme Inhib Med Chem. 2008 Feb;23(1):82-6. doi: 10.1080/14756360701421328. J Enzyme Inhib Med Chem. 2008. PMID: 18341258
-
Synthesis and Biological Activities of Oxadiazole Derivatives: A Review.Mini Rev Med Chem. 2016;16(10):825-45. doi: 10.2174/1389557516666160211120835. Mini Rev Med Chem. 2016. PMID: 26864552 Review.
-
Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents.Eur J Med Chem. 2015 Sep 18;102:487-529. doi: 10.1016/j.ejmech.2015.07.026. Epub 2015 Jul 18. Eur J Med Chem. 2015. PMID: 26310894 Review.
Cited by
-
Novel oxadiazole derivatives as potent inhibitors of α-amylase and α-glucosidase enzymes: Synthesis, in vitro evaluation, and molecular docking studies.Iran J Basic Med Sci. 2021 Dec;24(12):1632-1642. doi: 10.22038/IJBMS.2021.58429.12977. Iran J Basic Med Sci. 2021. PMID: 35432813 Free PMC article.
-
Substituted tetrazoles as multipurpose screening compounds.Mol Divers. 2017 Feb;21(1):9-27. doi: 10.1007/s11030-016-9711-x. Epub 2016 Dec 27. Mol Divers. 2017. PMID: 28028725
-
1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities.Molecules. 2021 Apr 7;26(8):2110. doi: 10.3390/molecules26082110. Molecules. 2021. PMID: 33916955 Free PMC article.
-
A fluorogenic aryl fluorosulfate for intraorganellar transthyretin imaging in living cells and in Caenorhabditis elegans.J Am Chem Soc. 2015 Jun 17;137(23):7404-14. doi: 10.1021/jacs.5b03042. Epub 2015 Jun 8. J Am Chem Soc. 2015. PMID: 26051248 Free PMC article.
-
General α-Amino 1,3,4-Oxadiazole Synthesis via Late-Stage Reductive Functionalization of Tertiary Amides and Lactams*.Angew Chem Int Ed Engl. 2021 Sep 1;60(36):19725-19729. doi: 10.1002/anie.202107536. Epub 2021 Aug 3. Angew Chem Int Ed Engl. 2021. PMID: 34191400 Free PMC article.
References
-
- Nagaraj, Chaluvaraju K.C., Niranjan M.S., Kiran S. 1,3,4-Oxadiazole: A potent drug candidate with various pharmacological activities. Int. J. Pharm. Pharm. Sci. 2011;3:9–16.
-
- Boström J., Hogner A., Llinàs A., Wellner E., Plowright A.T. Oxadiazoles in medicinal chemistry. J. Med. Chem. 2012;55:1817–1830. - PubMed
-
- James N.D., Growcott J.W. Zibotentan. Drugs Future. 2009;34:624–633.
-
- Scifinder Scholar. Criteria Used to Search: Research Topic: 1,3,4-Oxadiazole. [(accessed on 7 June 2012)]. Available online: http://www.cas.org/products/scifinder/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

