Nitric oxide-mediated maintenance of redox homeostasis contributes to NPR1-dependent plant innate immunity triggered by lipopolysaccharides
- PMID: 22926319
- PMCID: PMC3461531
- DOI: 10.1104/pp.112.201798
Nitric oxide-mediated maintenance of redox homeostasis contributes to NPR1-dependent plant innate immunity triggered by lipopolysaccharides
Abstract
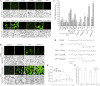
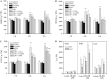
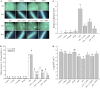
The perception of lipopolysaccharides (LPS) by plant cells can lead to nitric oxide (NO) production and defense gene induction. However, the signaling cascades underlying these cellular responses have not yet been resolved. This work investigated the biosynthetic origin of NO and the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 (NPR1) to gain insight into the mechanism involved in LPS-induced resistance of Arabidopsis (Arabidopsis thaliana). Analysis of inhibitors and mutants showed that LPS-induced NO synthesis was mainly mediated by an arginine-utilizing source of NO generation. Furthermore, LPS-induced NO caused transcript accumulation of alternative oxidase genes and increased antioxidant enzyme activity, which enhanced antioxidant capacity and modulated redox state. We also analyzed the subcellular localization of NPR1 to identify the mechanism for protein-modulated plant innate immunity triggered by LPS. LPS-activated defense responses, including callose deposition and defense-related gene expression, were found to be regulated through an NPR1-dependent pathway. In summary, a significant NO synthesis induced by LPS contributes to the LPS-induced defense responses by up-regulation of defense genes and modulation of cellular redox state. Moreover, NPR1 plays an important role in LPS-triggered plant innate immunity.
Figures






Similar articles
-
Regulatory role of nitric oxide in lipopolysaccharides-triggered plant innate immunity.Plant Signal Behav. 2013 Jan;8(1):e22554. doi: 10.4161/psb.22554. Epub 2012 Dec 6. Plant Signal Behav. 2013. PMID: 23221762 Free PMC article.
-
Crosstalk between nitric oxide and glutathione is required for NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1)-dependent defense signaling in Arabidopsis thaliana.New Phytol. 2015 Nov;208(3):860-72. doi: 10.1111/nph.13502. Epub 2015 Jun 10. New Phytol. 2015. PMID: 26096525
-
Novel molecular components involved in callose-mediated Arabidopsis defense against Salmonella enterica and Escherichia coli O157:H7.BMC Plant Biol. 2020 Jan 8;20(1):16. doi: 10.1186/s12870-019-2232-x. BMC Plant Biol. 2020. PMID: 31914927 Free PMC article.
-
NPR1 in JazzSet with Pathogen Effectors.Trends Plant Sci. 2018 Jun;23(6):469-472. doi: 10.1016/j.tplants.2018.04.007. Epub 2018 May 9. Trends Plant Sci. 2018. PMID: 29753632 Review.
-
The functions of WHIRLY1 and REDOX-RESPONSIVE TRANSCRIPTION FACTOR 1 in cross tolerance responses in plants: a hypothesis.Philos Trans R Soc Lond B Biol Sci. 2014 Mar 3;369(1640):20130226. doi: 10.1098/rstb.2013.0226. Print 2014 Apr 19. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24591713 Free PMC article. Review.
Cited by
-
Burkholderia phytofirmans PsJN Confers Grapevine Resistance against Botrytis cinerea via a Direct Antimicrobial Effect Combined with a Better Resource Mobilization.Front Plant Sci. 2016 Aug 23;7:1236. doi: 10.3389/fpls.2016.01236. eCollection 2016. Front Plant Sci. 2016. PMID: 27602036 Free PMC article.
-
Quercetin Alleviates Lipopolysaccharide-Induced Inflammatory Response in Bovine Mammary Epithelial Cells by Suppressing TLR4/NF-κB Signaling Pathway.Front Vet Sci. 2022 Jul 4;9:915726. doi: 10.3389/fvets.2022.915726. eCollection 2022. Front Vet Sci. 2022. PMID: 35865878 Free PMC article.
-
Lipopolysaccharides Trigger Two Successive Bursts of Reactive Oxygen Species at Distinct Cellular Locations.Plant Physiol. 2018 Mar;176(3):2543-2556. doi: 10.1104/pp.17.01637. Epub 2018 Feb 5. Plant Physiol. 2018. PMID: 29431629 Free PMC article.
-
Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants.Int J Mol Sci. 2013 Mar 26;14(4):6805-47. doi: 10.3390/ijms14046805. Int J Mol Sci. 2013. PMID: 23531539 Free PMC article. Review.
-
Lipopolysaccharide-induced priming enhances NO-mediated activation of defense responses in pearl millet challenged with Sclerospora graminicola.3 Biotech. 2018 Nov;8(11):475. doi: 10.1007/s13205-018-1501-y. Epub 2018 Nov 10. 3 Biotech. 2018. PMID: 30456009 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

