Tumor cell migration in complex microenvironments
- PMID: 22926411
- PMCID: PMC3557537
- DOI: 10.1007/s00018-012-1115-1
Tumor cell migration in complex microenvironments
Abstract
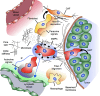
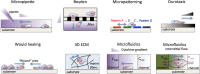
Tumor cell migration is essential for invasion and dissemination from primary solid tumors and for the establishment of lethal secondary metastases at distant organs. In vivo and in vitro models enabled identification of different factors in the tumor microenvironment that regulate tumor progression and metastasis. However, the mechanisms by which tumor cells integrate these chemical and mechanical signals from multiple sources to navigate the complex microenvironment remain poorly understood. In this review, we discuss the factors that influence tumor cell migration with a focus on the migration of transformed carcinoma cells. We provide an overview of the experimental and computational methods that allow the investigation of tumor cell migration, and we highlight the benefits and shortcomings of the various assays. We emphasize that the chemical and mechanical stimulus paradigms are not independent and that crosstalk between them motivates the development of new assays capable of applying multiple, simultaneous stimuli and imaging the cellular migratory response in real-time. These next-generation assays will more closely mimic the in vivo microenvironment to provide new insights into tumor progression, inform techniques to control tumor cell migration, and render cancer more treatable.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

