Histone methylase MLL1 has critical roles in tumor growth and angiogenesis and its knockdown suppresses tumor growth in vivo
- PMID: 22926525
- PMCID: PMC3511651
- DOI: 10.1038/onc.2012.352
Histone methylase MLL1 has critical roles in tumor growth and angiogenesis and its knockdown suppresses tumor growth in vivo
Abstract
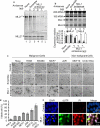
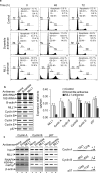
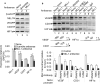
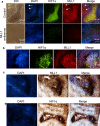
Mixed lineage leukemias (MLLs) are human histone H3 lysine-4-specific methyl transferases that have critical roles in gene expression, epigenetics and cancer. Herein, we demonstrated that antisense-mediated knockdown of MLL1 induced cell-cycle arrest and apoptosis in cultured cells. Intriguingly, application of MLL1 antisense specifically knocked down MLL1 in vivo and suppressed the growth of xenografted cervical tumor implanted in nude mouse. MLL1 knockdown downregulated various growth and angiogenic factors, such as HIF1α, VEGF and CD31, in tumor tissue affecting tumor growth. MLL1 is overexpressed along the line of vascular network and localized adjacent to endothelial cell layer expressing CD31, indicating potential roles of MLL1 in vasculogenesis. MLL1 is also overexpressed in the hypoxic regions along with HIF1α. Overall, our studies demonstrated that MLL1 is a key factor in hypoxia signaling, vasculogenesis and tumor growth, and its depletion suppresses tumor growth in vivo, indicating its potential in novel cancer therapy.
Figures



References
-
- Corey DR. Telomerase inhibition, oligonucleotides, and clinical trials. Oncogene. 2002 Jan 21;21(4):631–7. - PubMed
-
- Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer. 2005 Jun;5(6):468–79. - PubMed
-
- Lu B, Mu Y, Cao C, Zeng F, Schneider S, Tan J, et al. Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res. 2004 Apr 15;64(8):2840–5. - PubMed
-
- Ryan BM, O'Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009 Nov;35(7):553–62. - PubMed
-
- Halatsch ME, Schmidt U, Behnke-Mursch J, Unterberg A, Wirtz CR. Epidermal growth factor receptor inhibition for the treatment of glioblastoma multiforme and other malignant brain tumours. Cancer Treat Rev. 2006 Apr;32(2):74–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

