CD24 is an effector of HIF-1-driven primary tumor growth and metastasis
- PMID: 22926560
- PMCID: PMC3488144
- DOI: 10.1158/0008-5472.CAN-11-3666
CD24 is an effector of HIF-1-driven primary tumor growth and metastasis
Abstract
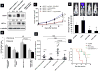
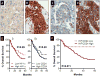
Hypoxia drives malignant progression in part by promoting accumulation of the oncogenic transcription factor hypoxia inducible factor-1α (HIF-1α) in tumor cells. Tumor aggressiveness also relates to elevation of the cancer stem cell-associated membrane protein CD24, which has been causally implicated in tumor formation and metastasis in experimental models. Here, we link these two elements by showing that hypoxia induces CD24 expression through a functional hypoxia responsive element in the CD24 promoter. HIF-1α overexpression induced CD24 mRNA and protein under normoxic conditions, with this effect traced to a recruitment of endogenous HIF-1α to the CD24 promoter. Short hairpin RNA-mediated attenuation of HIF-1α or CD24 expression reduced cancer cell survival in vitro and in vivo at the levels of primary and metastatic tumor growth. CD24 overexpression in HIF-1α-depleted cancer cells rescued this decrease, whereas HIF-1α overexpression in CD24-depleted cells did not. Analysis of clinical tumor specimens revealed a correlation between HIF-1α and CD24 levels and an association of their coexpression to decreased patient survival. Our results establish a mechanistic linkage between 2 critically important molecules in cancer, identifying CD24 as a critical HIF-1α transcriptional target and biologic effector, strengthening the rationale to target CD24 for cancer therapy.
©2012 AACR.
Conflict of interest statement
Figures





References
-
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. - PubMed
-
- Sutherland RM. Tumor hypoxia and gene expression--implications for malignant progression and therapy. Acta Oncol. 1998;37:567–74. - PubMed
-
- Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26:319–31. - PubMed
-
- Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

