Epithelial permeability alterations in an in vitro air-liquid interface model of allergic fungal rhinosinusitis
- PMID: 22927233
- PMCID: PMC3511593
- DOI: 10.1002/alr.21077
Epithelial permeability alterations in an in vitro air-liquid interface model of allergic fungal rhinosinusitis
Abstract
Background: Chronic rhinosinusitis (CRS) is an inflammatory upper-airway disease with numerous etiologies. Patients with a characteristic subtype of CRS, allergic fungal rhinosinusitis (AFRS), display increased expression of T helper 2 (Th2) cytokines and antigen-specific immunoglobulin E (IgE). Various sinonasal inflammatory conditions are associated with alterations in epithelial barrier function. The aim of this study was to compare epithelial permeability and intercellular junctional protein expression among cultured primary sinonasal cells from AFRS patients vs noninflammatory controls.
Methods: Epithelial cells isolated from paranasal sinus mucosa of AFRS and noninflammatory control patients were grown to confluence on permeable supports and transitioned to air-liquid interface (ALI). Transepithelial resistance (TER) was measured with a horizontal Ussing chamber to characterize the functional permeability of each cell type. After TER recordings were complete, a panel of intercellular junctional proteins was assessed by Western blot and immunofluorescence labeling followed by confocal microscopy.
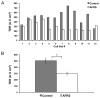
Results: After 12 samples were measured from each group, we observed a 41% mean decrease in TER in AFRS cells (296 ± 89 ohms × cm(2) ) compared to control (503 ± 134 ohms × cm(2) , p = 0.006). TER deficits observed in AFRS were associated with decreased expression of the tight junction proteins occludin and junctional adhesion molecule-A (JAM-A), and increased expression of a leaky tight junction protein claudin-2.
Conclusion: Cultured sinonasal epithelium from AFRS patients displayed increased epithelial permeability and altered expression of intercellular junctional proteins. Given that these cells were not incubated with inflammatory cytokines in vitro, the cultured AFRS epithelial alterations may represent a retained modification in protein expression from the in vivo phenotype.
Copyright © 2013 American Rhinologic Society-American Academy of Otolaryngic Allergy, LLC.
Conflict of interest statement
Conflict of Interest Disclosure:
[Table: see text]
Figures


References
-
- Stephenson MF, Mfuna L, Dowd SE, et al. Molecular characterization of the polymicrobial flora in chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2010;39:182–7. - PubMed
-
- Bent JP, 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–8. - PubMed
-
- Bateman ND, Fahy C, Woolford TJ. Nasal polyps: still more questions than answers. J Laryngol Otol. 2003;117:1–9. - PubMed
-
- Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: Developing guidance for clinical trials. Otolaryngol Head Neck Surg. 2006;135:S31–80. - PubMed
-
- Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22:85–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK072564/DK/NIDDK NIH HHS/United States
- KL2 TR000455/TR/NCATS NIH HHS/United States
- T35-HL007473/HL/NHLBI NIH HHS/United States
- DK061379/DK/NIDDK NIH HHS/United States
- R01 DK072564/DK/NIDDK NIH HHS/United States
- KL2 RR025009/RR/NCRR NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- T35 HL007473/HL/NHLBI NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- R01 DK059888/DK/NIDDK NIH HHS/United States
- R01 DK061379/DK/NIDDK NIH HHS/United States
- DK059888/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

