Fluvastatin inhibits FLT3 glycosylation in human and murine cells and prolongs survival of mice with FLT3/ITD leukemia
- PMID: 22927251
- PMCID: PMC3471516
- DOI: 10.1182/blood-2012-01-403493
Fluvastatin inhibits FLT3 glycosylation in human and murine cells and prolongs survival of mice with FLT3/ITD leukemia
Abstract
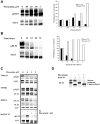
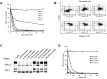
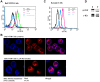
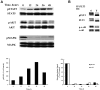
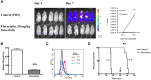
FLT3 is frequently mutated in acute myeloid leukemia (AML), but resistance has limited the benefit of tyrosine kinase inhibitors (TKI). We demonstrate that statins can impair FLT3 glycosylation, thus leading to loss of surface expression and induction of cell death, as well as mitigation of TKI resistance. Immunofluorescence microscopy confirms a reduction in surface localization and an increase in intracellular FLT3/internal tandem duplication (ITD) accumulation. This aberrant localization was associated with increased STAT5 activation but inhibition of both MAPK and AKT phosphorylation. Growth inhibition studies indicate that FLT3/ITD-expressing cells were killed with an IC(50) within a range of 0.2-2μM fluvastatin. Several mechanisms of resistance could be circumvented by fluvastatin treatment. An increase in the IC(50) for inhibition of phosphorylated FLT3/ITD by lestaurtinib caused by exogenous FLT3 ligand, resistance to sorafenib caused by the D835Y or FLT3/ITD N676K mutations, and activation of the IL-3 compensatory pathway were all negated by fluvastatin treatment. Finally, fluvastatin treatment in vivo reduced engraftment of BaF3 FLT3/ITD cells in Balb/c mice. These results demonstrate that statins, a class of drugs already approved by the US Food and Drug Administration, might be repurposed for the management of FLT3 mutant acute myeloid leukemia cases either alone or in conjunction with FLT3 TKI.
Figures


References
-
- Reilly JT. FLT3 and its role in the pathogenesis of acute myeloid leukaemia. Leuk Lymphoma. 2003;44(1):1–7. - PubMed
-
- Lyman SD, James L, Vanden Bos T, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75(6):1157–1167. - PubMed
-
- Lavagna-Sevenier C, Marchetto S, Birnbaum D, Rosnet O. FLT3 signaling in hematopoietic cells involves CBL, SHC and an unknown P115 as prominent tyrosine-phosphorylated substrates. Leukemia. 1998;12(3):301–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

