Using lectins in biomarker research: addressing the limitations of sensitivity and availability
- PMID: 22927350
- PMCID: PMC3703587
- DOI: 10.1002/prca.201200014
Using lectins in biomarker research: addressing the limitations of sensitivity and availability
Abstract
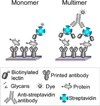
Carbohydrates have fundamental roles throughout biology, yet they have not been as well studied as proteins and nucleic acids, in part due to limitations in the experimental tools. Improved methods for studying glycans could spur significant advances in the understanding and application of glycobiology. The use of affinity reagents, such as lectins and glycan-binding antibodies, is a valuable complement to methods involving mass spectrometry and chromatography. This article addresses two limitations that have prevented the broader experimental use of glycan-binding proteins: sensitivity and availability. The sensitivity limitation stems from the poor affinity that many glycan-binding proteins have as isolated analytical reagents. To address this problem, I propose making use of multivalent interactions between lectins and glycans, mimicking those frequently found in the biological setting. Recent experiments show that a practical technique for producing lectin multimers can significantly improve detection sensitivity. The second limitation, availability, is the difficulty of finding and obtaining glycan-binding proteins that recognize less common or arbitrarily defined glycan structures. To address this problem, I propose translating the wealth of existing glycan array data into a quantitative, searchable database of the specificities of glycan-binding proteins. Such a resource would allow us to more easily identify proteins with defined specificities and perform detailed comparisons between reagents. Solutions to these two limitations could lead to the more effective use of, and a broader range of, glycan-binding reagents.
© 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

Similar articles
-
Global comparisons of lectin-glycan interactions using a database of analyzed glycan array data.Mol Cell Proteomics. 2013 Apr;12(4):1026-35. doi: 10.1074/mcp.M112.026641. Epub 2013 Feb 11. Mol Cell Proteomics. 2013. PMID: 23399549 Free PMC article.
-
Modulation of glycan detection on specific glycoproteins by lectin multimerization.Anal Chem. 2013 Feb 5;85(3):1689-98. doi: 10.1021/ac302826a. Epub 2013 Jan 16. Anal Chem. 2013. PMID: 23286506 Free PMC article.
-
The detection and discovery of glycan motifs in biological samples using lectins and antibodies: new methods and opportunities.Adv Cancer Res. 2015;126:167-202. doi: 10.1016/bs.acr.2014.11.003. Epub 2015 Feb 7. Adv Cancer Res. 2015. PMID: 25727148 Free PMC article. Review.
-
LectinOracle: A Generalizable Deep Learning Model for Lectin-Glycan Binding Prediction.Adv Sci (Weinh). 2022 Jan;9(1):e2103807. doi: 10.1002/advs.202103807. Epub 2021 Dec 4. Adv Sci (Weinh). 2022. PMID: 34862760 Free PMC article.
-
Concept, strategy and realization of lectin-based glycan profiling.J Biochem. 2008 Aug;144(2):139-47. doi: 10.1093/jb/mvn043. Epub 2008 Apr 4. J Biochem. 2008. PMID: 18390573 Review.
Cited by
-
Plasma glycomics predict cardiovascular disease in patients with ART-controlled HIV infections.FASEB J. 2019 Feb;33(2):1852-1859. doi: 10.1096/fj.201800923R. Epub 2018 Sep 5. FASEB J. 2019. PMID: 30183373 Free PMC article.
-
LeGenD: determining N-glycoprofiles using an explainable AI-leveraged model with lectin profiling.bioRxiv [Preprint]. 2024 Mar 30:2024.03.27.587044. doi: 10.1101/2024.03.27.587044. bioRxiv. 2024. PMID: 38585977 Free PMC article. Preprint.
-
High-Throughput Glycomic Methods.Chem Rev. 2022 Oct 26;122(20):15865-15913. doi: 10.1021/acs.chemrev.1c01031. Epub 2022 Jul 7. Chem Rev. 2022. PMID: 35797639 Free PMC article. Review.
-
A comparative study of glycoproteomes in androgen-sensitive and -independent prostate cancer cell lines.Mol Cell Biochem. 2014 Jan;386(1-2):189-98. doi: 10.1007/s11010-013-1857-6. Epub 2013 Oct 9. Mol Cell Biochem. 2014. PMID: 24104455 Free PMC article.
-
Development of an exoglycosidase plate-based assay for detecting α1-3,4 fucosylation biomarker in individuals with HNF1A-MODY.Glycobiology. 2022 Mar 30;32(3):230-238. doi: 10.1093/glycob/cwab107. Glycobiology. 2022. PMID: 34939081 Free PMC article.
References
-
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. - PubMed
-
- Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochimica et biophysica acta. 2011 - PubMed
-
- Rudiger H, Gabius HJ. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconjugate journal. 2001;18:589–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

