Pathogenic bacterium Helicobacter pylori alters the expression profile of p53 protein isoforms and p53 response to cellular stresses
- PMID: 22927405
- PMCID: PMC3458371
- DOI: 10.1073/pnas.1205664109
Pathogenic bacterium Helicobacter pylori alters the expression profile of p53 protein isoforms and p53 response to cellular stresses
Abstract
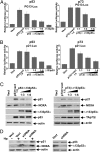
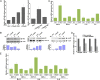
The p53 protein plays a central role in the prevention of tumorigenesis. Cellular stresses, such as DNA damage and aberrant oncogene activation, trigger induction of p53 that halts cellular proliferation and allows cells to be repaired. If cellular damage is beyond the capability of the repair mechanisms, p53 induces apoptosis or cell cycle arrest, preventing damaged cells from becoming cancerous. However, emerging evidence suggests that the function of p53 needs to be considered as isoform-specific. Here, we report that the expression profile of p53 can be shifted toward inhibitory p53 isoforms by the pathogenic bacterium Helicobacter pylori, which is known for its strong association with gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma. We found that interaction of H. pylori with gastric epithelial cells, mediated via the cag pathogenicity island, induces N-terminally truncated Δ133p53 and Δ160p53 isoforms in human cells. Induction of an orthologous p53 isoform, Δ153p53, was also found in H. pylori-infected Mongolian gerbils. The p53 isoforms inhibit p53 and p73 activities, induce NF-κB, and increase survival of infected cells. Expression of Δ133p53, in response to H. pylori infection, is regulated by phosphorylation of c-Jun and activation of activator protein-1-dependent transcription. Together, these results provide unique insights into the regulation of p53 protein and may contribute to the understanding of tumorigenesis associated with H. pylori.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Regulation of p53 tumor suppressor by Helicobacter pylori in gastric epithelial cells.Gastroenterology. 2010 Oct;139(4):1333-43. doi: 10.1053/j.gastro.2010.06.018. Epub 2010 Jun 12. Gastroenterology. 2010. PMID: 20547161 Free PMC article.
-
Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island.J Biol Chem. 1999 Oct 29;274(44):31655-62. doi: 10.1074/jbc.274.44.31655. J Biol Chem. 1999. PMID: 10531374
-
NF-kappaB activation by Helicobacter pylori requires Akt-mediated phosphorylation of p65.BMC Microbiol. 2009 Feb 12;9:36. doi: 10.1186/1471-2180-9-36. BMC Microbiol. 2009. Retraction in: BMC Microbiol. 2011 Jun 02;11:128. doi: 10.1186/1471-2180-11-128. PMID: 19216748 Free PMC article. Retracted.
-
Helicobacter pylori and gastric carcinoma--from the view point of animal model.Keio J Med. 2002 Dec;51 Suppl 2:69-73. doi: 10.2302/kjm.51.supplement2_69. Keio J Med. 2002. PMID: 12528942 Review.
-
Helicobacter pylori and mucosa-associated lymphoid tissue: what's new.Hematology Am Soc Hematol Educ Program. 2013;2013:109-17. doi: 10.1182/asheducation-2013.1.109. Hematology Am Soc Hematol Educ Program. 2013. PMID: 24319171 Review.
Cited by
-
p53 protein isoforms: key regulators in the front line of pathogen infections?PLoS Pathog. 2013;9(4):e1003246. doi: 10.1371/journal.ppat.1003246. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23592981 Free PMC article. No abstract available.
-
Adaptive homeostasis and the p53 isoform network.EMBO Rep. 2021 Dec 6;22(12):e53085. doi: 10.15252/embr.202153085. Epub 2021 Nov 15. EMBO Rep. 2021. PMID: 34779563 Free PMC article. Review.
-
Interplay between DNA repair and inflammation, and the link to cancer.Crit Rev Biochem Mol Biol. 2014 Mar-Apr;49(2):116-39. doi: 10.3109/10409238.2013.875514. Epub 2014 Jan 13. Crit Rev Biochem Mol Biol. 2014. PMID: 24410153 Free PMC article. Review.
-
Δ133p53α enhances metabolic and cellular fitness of TCR-engineered T cells and promotes superior antitumor immunity.J Immunother Cancer. 2021 Jun;9(6):e001846. doi: 10.1136/jitc-2020-001846. J Immunother Cancer. 2021. PMID: 34112738 Free PMC article.
-
p53 Isoforms and Their Implications in Cancer.Cancers (Basel). 2018 Aug 25;10(9):288. doi: 10.3390/cancers10090288. Cancers (Basel). 2018. PMID: 30149602 Free PMC article. Review.
References
-
- Levine AJ, Hu W, Feng Z. The P53 pathway: What questions remain to be explored? Cell Death Differ. 2006;13:1027–1036. - PubMed
-
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. - PubMed
-
- Matsumoto Y, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

