An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis
- PMID: 22927409
- PMCID: PMC3443174
- DOI: 10.1073/pnas.1208650109
An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis
Abstract
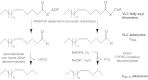
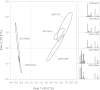
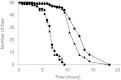
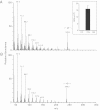
Insects use hydrocarbons as cuticular waterproofing agents and as contact pheromones. Although their biosynthesis from fatty acyl precursors is well established, the last step of hydrocarbon biosynthesis from long-chain fatty aldehydes has remained mysterious. We show here that insects use a P450 enzyme of the CYP4G family to oxidatively produce hydrocarbons from aldehydes. Oenocyte-directed RNAi knock-down of Drosophila CYP4G1 or NADPH-cytochrome P450 reductase results in flies deficient in cuticular hydrocarbons, highly susceptible to desiccation, and with reduced viability upon adult emergence. The heterologously expressed enzyme converts C(18)-trideuterated octadecanal to C(17)-trideuterated heptadecane, showing that the insect enzyme is an oxidative decarbonylase that catalyzes the cleavage of long-chain aldehydes to hydrocarbons with the release of carbon dioxide. This process is unlike cyanobacteria that use a nonheme diiron decarbonylase to make alkanes from aldehydes with the release of formate. The unique and highly conserved insect CYP4G enzymes are a key evolutionary innovation that allowed their colonization of land.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gibbs AG. Water-proofing properties of cuticular lipids. Am Zool. 1998;38:471–482.
-
- Howard RW, Blomquist GJ. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol. 2005;50:371–393. - PubMed
-
- Schirmer A, Rude MA, Li X, Popova E, del Cardayre SB. Microbial biosynthesis of alkanes. Science. 2010;329:559–562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

