Identification of a novel role for dematin in regulating red cell membrane function by modulating spectrin-actin interaction
- PMID: 22927433
- PMCID: PMC3471709
- DOI: 10.1074/jbc.M111.305441
Identification of a novel role for dematin in regulating red cell membrane function by modulating spectrin-actin interaction
Abstract
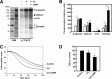
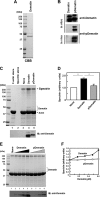
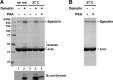
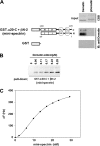
The membrane skeleton plays a central role in maintaining the elasticity and stability of the erythrocyte membrane, two biophysical features critical for optimal functioning and survival of red cells. Many constituent proteins of the membrane skeleton are phosphorylated by various kinases, and phosphorylation of β-spectrin by casein kinase and of protein 4.1R by PKC has been documented to modulate erythrocyte membrane mechanical stability. In this study, we show that activation of endogenous PKA by cAMP decreases membrane mechanical stability and that this effect is mediated primarily by phosphorylation of dematin. Co-sedimentation assay showed that dematin facilitated interaction between spectrin and F-actin, and phosphorylation of dematin by PKA markedly diminished this activity. Quartz crystal microbalance measurement revealed that purified dematin specifically bound the tail region of the spectrin dimer in a saturable manner with a submicromolar affinity. Pulldown assay using recombinant spectrin fragments showed that dematin, but not phospho-dematin, bound to the tail region of the spectrin dimer. These findings imply that dematin contributes to the maintenance of erythrocyte membrane mechanical stability by facilitating spectrin-actin interaction and that phosphorylation of dematin by PKA can modulate these effects. In this study, we have uncovered a novel functional role for dematin in regulating erythrocyte membrane function.
Figures
References
-
- Mohandas N., Chasis J. A. (1993) Red blood cell deformability, membrane material properties, and shape: regulation by transmembrane, skeletal, and cytosolic proteins and lipids. Semin. Hematol. 30, 171–192 - PubMed
-
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. (1979) In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature 280, 811–814 - PubMed
-
- Gardner K., Bennett V. (1987) Modulation of spectrin-actin assembly by erythrocyte adducin. Nature 328, 359–362 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

