Kcnh1 voltage-gated potassium channels are essential for early zebrafish development
- PMID: 22927438
- PMCID: PMC3471731
- DOI: 10.1074/jbc.M112.363978
Kcnh1 voltage-gated potassium channels are essential for early zebrafish development
Abstract
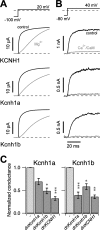
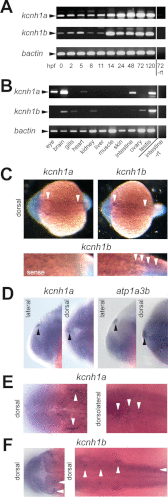
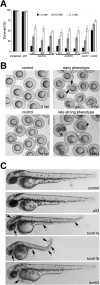
The Kcnh1 gene encodes a voltage-gated potassium channel highly expressed in neurons and involved in tumor cell proliferation, yet its physiological roles remain unclear. We have used the zebrafish as a model to analyze Kcnh1 function in vitro and in vivo. We found that the kcnh1 gene is duplicated in teleost fish (i.e. kcnh1a and kcnh1b) and that both genes are maternally expressed during early development. In adult zebrafish, kcnh1a and kcnh1b have distinct expression patterns but share expression in brain and testis. Heterologous expression of both genes in Xenopus oocytes revealed a strong conservation of characteristic functional properties between human and fish channels, including a unique sensitivity to intracellular Ca(2+)/calmodulin and modulation of voltage-dependent gating by extracellular Mg(2+). Using a morpholino antisense approach, we demonstrate a strong kcnh1 loss-of-function phenotype in developing zebrafish, characterized by growth retardation, delayed hindbrain formation, and embryonic lethality. This late phenotype was preceded by transcriptional up-regulation of known cell-cycle inhibitors (p21, p27, cdh2) and down-regulation of pro-proliferative factors, including cyclin D1, at 70% epiboly. These results reveal an unanticipated basic activity of kcnh1 that is crucial for early embryonic development and patterning.
Figures



References
-
- Ganetzky B., Wu C. F. (1983) Neurogenetic analysis of potassium currents in Drosophila. Synergistic effects on neuromuscular transmission in double mutants. J. Neurogenet. 1, 17–28 - PubMed
-
- Ludwig J., Weseloh R., Karschin C., Liu Q., Netzer R., Engeland B., Stansfeld C., Pongs O. (2000) Cloning and functional expression of rat eag2, a new member of the ether-à-go-go family of potassium channels and comparison of its distribution with that of eag1. Mol. Cell. Neurosci. 16, 59–70 - PubMed
-
- Martin S., Lino de Oliveira C., Mello de Queiroz F., Pardo L. A., Stühmer W., Del Bel E. (2008) Eag1 potassium channel immunohistochemistry in the CNS of adult rat and selected regions of human brain. Neuroscience 155, 833–844 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

