Spectroscopic and kinetic investigation of the fully reduced and mixed valence states of ba3-cytochrome c oxidase from Thermus thermophilus: a Fourier transform infrared (FTIR) and time-resolved step-scan FTIR study
- PMID: 22927441
- PMCID: PMC3481344
- DOI: 10.1074/jbc.M112.403600
Spectroscopic and kinetic investigation of the fully reduced and mixed valence states of ba3-cytochrome c oxidase from Thermus thermophilus: a Fourier transform infrared (FTIR) and time-resolved step-scan FTIR study
Abstract
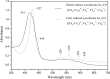
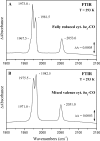
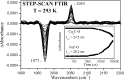
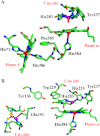
The complete understanding of a molecular mechanism of action requires the thermodynamic and kinetic characterization of different states and intermediates. Cytochrome c oxidase reduces O(2) to H(2)O, a reaction coupled to proton translocation across the membrane. Therefore, it is necessary to undertake a thorough characterization of the reduced form of the enzyme and the determination of the electron transfer processes and pathways between the redox-active centers. In this study Fourier transform infrared (FTIR) and time-resolved step-scan FTIR spectroscopy have been applied to study the fully reduced and mixed valence states of cytochrome ba(3) from Thermus thermophilus. We used as probe carbon monoxide (CO) to characterize both thermodynamically and kinetically the cytochrome ba(3)-CO complex in the 5.25-10.10 pH/pD range and to study the reverse intramolecular electron transfer initiated by the photolysis of CO in the two-electron reduced form. The time-resolved step-scan FTIR data revealed no pH/pD dependence in both the decay of the transient Cu(B)(1+)-CO complex and rebinding to heme a(3) rates, suggesting that no structural change takes place in the vicinity of the binuclear center. Surprisingly, photodissociation of CO from the mixed valence form of the enzyme does not lead to reverse electron transfer from the reduced heme a(3) to the oxidized low-spin heme b, as observed in all the other aa(3) and bo(3) oxidases previously examined. The heme b-heme a(3) electron transfer is guaranteed, and therefore, there is no need for structural rearrangements and complex synchronized cooperativities. Comparison among the available structures of ba(3)- and aa(3)-cytochrome c oxidases identifies possible active pathways involved in the electron transfer processes and key structural elements that contribute to the different behavior observed in cytochrome ba(3).
Figures
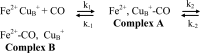


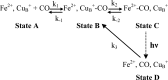
References
-
- Marcus R. A., Sutin N. (1985) Electron transfers in chemistry and biology. Biochim. Biophys. Acta 811, 265–322
-
- Page C. C., Moser C. C., Chen X., Dutton P. L. (1999) Natural engineering principles of electron tunneling in biological oxidation-reduction. Nature 402, 47–52 - PubMed
-
- Page C. C., Moser C. C., Dutton P. L. (2003) Mechanism for electron transfer within and between proteins. Curr. Opin. Chem. Biol. 7, 551–556 - PubMed
-
- Moser C. C., Page C. C., Farid R., Dutton P. L. (1995) Biological electron transfer. J. Bioenerg. Biomembr. 27, 263–274 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

