SUMOylated SoxE factors recruit Grg4 and function as transcriptional repressors in the neural crest
- PMID: 22927467
- PMCID: PMC3432773
- DOI: 10.1083/jcb.201204161
SUMOylated SoxE factors recruit Grg4 and function as transcriptional repressors in the neural crest
Abstract
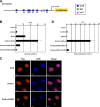
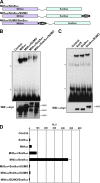
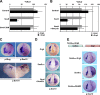
A growing number of transcriptional regulatory proteins are known to be modified by the small ubiquitin-like protein, SUMO. Posttranslational modification by SUMO may be one means by which transcriptional regulatory factors that play context-dependent roles in multiple processes can be regulated such that they direct the appropriate cellular and developmental outcomes. In early vertebrate embryos, SUMOylation of SoxE transcription factors profoundly affects their function, inhibiting their neural crest-inducing activity and promoting ear formation. In this paper, we provide mechanistic insight into how SUMO modification modulates SoxE function. We show that SUMOylation dramatically altered recruitment of transcriptional coregulator factors by SoxE proteins, displacing coactivators CREB-binding protein/p300 while promoting the recruitment of a corepressor, Grg4. These data demonstrate that SoxE proteins can function as transcriptional repressors in a SUMO-dependent manner. They further suggest a novel multivalent mechanism for SUMO-mediated recruitment of transcriptional coregulatory factors.
Figures




Similar articles
-
SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation.Dev Cell. 2005 Nov;9(5):593-603. doi: 10.1016/j.devcel.2005.09.016. Dev Cell. 2005. PMID: 16256735
-
P300 transcriptional repression is mediated by SUMO modification.Mol Cell. 2003 Apr;11(4):1043-54. doi: 10.1016/s1097-2765(03)00141-2. Mol Cell. 2003. PMID: 12718889
-
SUMO promotes HDAC-mediated transcriptional repression.Mol Cell. 2004 Feb 27;13(4):611-7. doi: 10.1016/s1097-2765(04)00060-7. Mol Cell. 2004. PMID: 14992729
-
SoxE factors as multifunctional neural crest regulatory factors.Int J Biochem Cell Biol. 2010 Mar;42(3):441-4. doi: 10.1016/j.biocel.2009.11.014. Epub 2009 Nov 30. Int J Biochem Cell Biol. 2010. PMID: 19931641 Free PMC article. Review.
-
Something about SUMO inhibits transcription.Curr Opin Genet Dev. 2005 Oct;15(5):536-41. doi: 10.1016/j.gde.2005.07.004. Curr Opin Genet Dev. 2005. PMID: 16095902 Review.
Cited by
-
Expression atlas of avian neural crest proteins: Neurulation to migration.Dev Biol. 2022 Mar;483:39-57. doi: 10.1016/j.ydbio.2021.12.018. Epub 2022 Jan 4. Dev Biol. 2022. PMID: 34990731 Free PMC article.
-
SUMOylation of FOXM1B alters its transcriptional activity on regulation of MiR-200 family and JNK1 in MCF7 human breast cancer cells.Int J Mol Sci. 2014 Jun 10;15(6):10233-51. doi: 10.3390/ijms150610233. Int J Mol Sci. 2014. PMID: 24918286 Free PMC article.
-
FOXP3 Activates SUMO-Conjugating UBC9 Gene in MCF7 Breast Cancer Cells.Int J Mol Sci. 2018 Jul 13;19(7):2036. doi: 10.3390/ijms19072036. Int J Mol Sci. 2018. PMID: 30011797 Free PMC article.
-
Transcriptome analysis reveals novel players in the cranial neural crest gene regulatory network.Genome Res. 2014 Feb;24(2):281-90. doi: 10.1101/gr.161182.113. Epub 2014 Jan 3. Genome Res. 2014. PMID: 24389048 Free PMC article.
-
SOX9 and the many facets of its regulation in the chondrocyte lineage.Connect Tissue Res. 2017 Jan;58(1):2-14. doi: 10.1080/03008207.2016.1183667. Epub 2016 Apr 29. Connect Tissue Res. 2017. PMID: 27128146 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

