Rad53 is essential for a mitochondrial DNA inheritance checkpoint regulating G1 to S progression
- PMID: 22927468
- PMCID: PMC3432762
- DOI: 10.1083/jcb.201205193
Rad53 is essential for a mitochondrial DNA inheritance checkpoint regulating G1 to S progression
Abstract
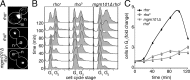
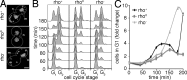
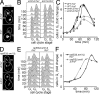
The Chk2-mediated deoxyribonucleic acid (DNA) damage checkpoint pathway is important for mitochondrial DNA (mtDNA) maintenance. We show in this paper that mtDNA itself affects cell cycle progression. Saccharomyces cerevisiae rho(0) cells, which lack mtDNA, were defective in G1- to S-phase progression. Deletion of subunit Va of cytochrome c oxidase, inhibition of F(1)F(0) adenosine triphosphatase, or replacement of all mtDNA-encoded genes with noncoding DNA did not affect G1- to S-phase progression. Thus, the cell cycle progression defect in rho(0) cells is caused by loss of DNA within mitochondria and not loss of respiratory activity or mtDNA-encoded genes. Rad53p, the yeast Chk2 homologue, was required for inhibition of G1- to S-phase progression in rho(0) cells. Pif1p, a DNA helicase and Rad53p target, underwent Rad53p-dependent phosphorylation in rho(0) cells. Thus, loss of mtDNA activated an established checkpoint kinase that inhibited G1- to S-phase progression. These findings support the existence of a Rad53p-regulated checkpoint that regulates G1- to S-phase progression in response to loss of mtDNA.
Figures


Similar articles
-
Differential involvement of the related DNA helicases Pif1p and Rrm3p in mtDNA point mutagenesis and stability.Gene. 2005 Jul 18;354:86-92. doi: 10.1016/j.gene.2005.03.031. Gene. 2005. PMID: 15907372
-
Linking DNA replication checkpoint to MBF cell-cycle transcription reveals a distinct class of G1/S genes.EMBO J. 2012 Apr 4;31(7):1798-810. doi: 10.1038/emboj.2012.27. Epub 2012 Feb 14. EMBO J. 2012. PMID: 22333912 Free PMC article.
-
The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae.Mol Biol Cell. 2005 Jun;16(6):3010-8. doi: 10.1091/mbc.e05-01-0053. Epub 2005 Apr 13. Mol Biol Cell. 2005. PMID: 15829566 Free PMC article.
-
The S-phase checkpoint: targeting the replication fork.Biol Cell. 2009 Aug 19;101(11):617-27. doi: 10.1042/BC20090053. Biol Cell. 2009. PMID: 19686094 Review.
-
Cell-cycle-specific activators of the Mec1/ATR checkpoint kinase.Biochem Soc Trans. 2011 Apr;39(2):600-5. doi: 10.1042/BST0390600. Biochem Soc Trans. 2011. PMID: 21428947 Review.
Cited by
-
Crosstalk between mitochondrial stress signals regulates yeast chronological lifespan.Mech Ageing Dev. 2014 Jan;135:41-9. doi: 10.1016/j.mad.2013.12.002. Epub 2013 Dec 25. Mech Ageing Dev. 2014. PMID: 24373996 Free PMC article.
-
Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease.Free Radic Biol Med. 2013 Sep;62:90-101. doi: 10.1016/j.freeradbiomed.2012.11.014. Epub 2012 Nov 29. Free Radic Biol Med. 2013. PMID: 23200807 Free PMC article.
-
Genetic instability in budding and fission yeast-sources and mechanisms.FEMS Microbiol Rev. 2015 Nov;39(6):917-67. doi: 10.1093/femsre/fuv028. Epub 2015 Jun 24. FEMS Microbiol Rev. 2015. PMID: 26109598 Free PMC article. Review.
-
Reciprocal interactions between mtDNA and lifespan control in budding yeast.Mol Biol Cell. 2019 Nov 15;30(24):2943-2952. doi: 10.1091/mbc.E18-06-0356. Epub 2019 Oct 10. Mol Biol Cell. 2019. PMID: 31599702 Free PMC article.
-
Mitochondrial membrane potential acts as a retrograde signal to regulate cell cycle progression.Life Sci Alliance. 2023 Sep 11;6(12):e202302091. doi: 10.26508/lsa.202302091. Print 2023 Dec. Life Sci Alliance. 2023. PMID: 37696576 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

