The autophagy-senescence connection in chemotherapy: must tumor cells (self) eat before they sleep?
- PMID: 22927544
- PMCID: PMC3500537
- DOI: 10.1124/jpet.112.197590
The autophagy-senescence connection in chemotherapy: must tumor cells (self) eat before they sleep?
Abstract
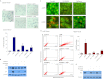
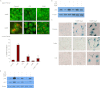
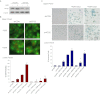
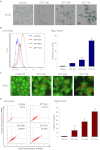
Exposure of MCF-7 breast tumor cells or HCT-116 colon carcinoma cells to clinically relevant concentrations of doxorubicin (Adriamycin; Farmitalia Research Laboratories, Milan, Italy) or camptothecin results in both autophagy and senescence. To determine whether autophagy is required for chemotherapy-induced senescence, reactive oxygen generation induced by Adriamycin was suppressed by N-acetyl cysteine and glutathione, and the induction of ataxia telangiectasia mutated, p53, and p21 was modulated pharmacologically and/or genetically. In all cases, autophagy and senescence were collaterally suppressed. The close association between autophagy and senescence indicated by these experiments reflects their collateral regulation via common signaling pathways. The potential relationship between autophagy and senescence was further examined through pharmacologic inhibition of autophagy with chloroquine and 3-methyl-adenine and genetic ablation of the autophagy-related genes ATG5 and ATG7. However, inhibition of autophagy by pharmacological and genetic approaches could not entirely abrogate the senescence response, which was only reduced and/or delayed. Taken together, our findings suggest that autophagy and senescence tend to occur in parallel, and furthermore that autophagy accelerates the development of the senescent phenotype. However, these responses are not inexorably linked or interdependent, as senescence can occur when autophagy is abrogated.
Figures








References
-
- Akbas SH, Timur M, Ozben T. (2005) The effect of quercetin on topotecan cytotoxicity in MCF-7 and MDA-MB 231 human breast cancer cells. J Surg Res 125:49–55 - PubMed
-
- Azad MB, Chen Y, Gibson SB. (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11:777–790 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

