Wnt antagonist SFRP1 functions as a secreted mediator of senescence
- PMID: 22927647
- PMCID: PMC3486147
- DOI: 10.1128/MCB.06023-11
Wnt antagonist SFRP1 functions as a secreted mediator of senescence
Abstract
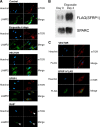
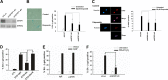
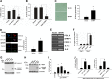
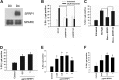
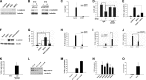
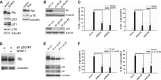
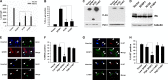
Cellular senescence has emerged as a critical tumor suppressive mechanism in recent years, but relatively little is known about how senescence occurs. Here, we report that secreted Frizzled-related protein 1 (SFRP1), a secreted antagonist of Wnt signaling, is oversecreted upon cellular senescence caused by DNA damage or oxidative stress. SFRP1 is necessary for stress-induced senescence caused by these factors and is sufficient for the induction of senescence phenotypes. We present evidence suggesting that SFRP1 functions as a secreted mediator of senescence through inhibition of Wnt signaling and activation of the retinoblastoma (Rb) pathway and that cancer-associated SFRP1 mutants are defective for senescence induction.
Figures

References
-
- Acosta JC, et al. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018 - PubMed
-
- Bartkova J, et al. 2006. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444: 633–637 - PubMed
-
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. 2008. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121: 737–746 - PubMed
-
- Caldwell GM, et al. 2004. The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Res. 64: 883–888 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
