Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy
- PMID: 22927737
- PMCID: PMC3422154
- DOI: 10.2147/OPTH.S31902
Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy
Abstract
Background: The purpose of this study was to evaluate nepafenac ophthalmic suspension 0.1% (Nevanac(®); Alcon Research Ltd) in the prevention of macular edema following cataract surgery in diabetic retinopathy patients.
Methods: This was a multicenter, randomized, double-masked, vehicle-controlled study of 263 adult diabetic patients with nonproliferative diabetic retinopathy requiring cataract surgery. Patients were randomized (1:1) to instill nepafenac or vehicle three times daily beginning 1 day prior to surgery through day 90. Efficacy included the percentage of patients who developed macular edema (≥30% increase in central subfield macular thickness from baseline) and the percentage of patients with decreases of more than five letters in best-corrected visual acuity from day 7 to 90.
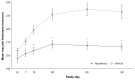
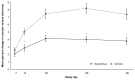
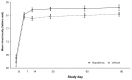
Results: A significantly lower percentage of patients in the nepafenac group developed macular edema relative to patients in the vehicle group (3.2% versus 16.7%; P < 0.001). A significantly lower percentage of patients in the nepafenac group had best-corrected visual acuity decreases of more than five letters relative to patients in the vehicle group on day 30 (P < 0.001), day 60 (P = 0.002), and day 90 (P = 0.006). The mean central subfield macular thickness and mean percent change from baseline in macular volume were also significantly lower in the nepafenac group versus the vehicle group at days 14 through 90 (P ≤ 0.005). No safety issues or trends were identified when dosing was increased to 90 days that negatively impacted the favorable benefit/risk profile of nepafenac.
Conclusion: Nepafenac demonstrated statistically significant and clinically relevant advantages compared with vehicle in preventing macular edema and maintaining visual acuity in diabetic patients following cataract surgery. These advantages were seen at multiple time points over the course of the 90-day therapy period. There was no clinically relevant increase in risk from 90 days dosing compared with 14 days. Therefore, with a similar safety profile and benefit in preventing macular edema and maintaining vision, the risk/benefit to the diabetic patient undergoing cataract surgery appears to be positive.
Keywords: cataract extraction; diabetes; macular edema; nonsteroidal anti-inflammatory drug; ocular surgery; retinopathy; topical.
Figures
References
-
- Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis T, Tsinopoulos I, Pavesio CE. Macular edema. Surv Ophthalmol. 2004;49:470–490. - PubMed
-
- Gallemore RP. NSAIDs in treatment of retinal disorders. Rev Ophth. 2006;13:81–88.
-
- Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team. Arch Ophthalmol. 1994;112:239–252. - PubMed
-
- Ursell PG, Spalton DJ, Whitcup SM, Nussenblatt RB. Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg. 1999;25:1492–1497. - PubMed
-
- Hayashi K, Igarashi C, Hirata A, Hayashi H. Changes in diabetic macular oedema after phacoemulsification surgery. Eye (Lond) 2009;23:389–396. - PubMed
LinkOut - more resources
Full Text Sources
Medical

