Characterization of outcomes 1 year after endoscopic thermal vapor ablation for patients with heterogeneous emphysema
- PMID: 22927751
- PMCID: PMC3422124
- DOI: 10.2147/COPD.S31082
Characterization of outcomes 1 year after endoscopic thermal vapor ablation for patients with heterogeneous emphysema
Abstract
Introduction: Endoscopic lung volume reduction has been developed as a therapeutic option for advanced emphysema. Six-month results following treatment with endoscopic thermal vapor ablation (InterVapor; Uptake Medical, Tustin, CA) were described previously, and here we report observations from the 12-month assessment.
Methods: Two multicenter, international, single-arm trials of InterVapor (unilateral upper lobe treatment) in patients with upper lobe predominant emphysema were conducted.
Inclusion criteria: forced expiratory volume in 1 second (FEV(1)) 15%-45% predicted, residual volume > 150%, total lung capacity > 100%, 6-minute walk distance (6MWD) > 140 m, and diffusing capacity for carbon monoxide > 20% predicted. Efficacy endpoints: spirometry, body plethysmography, lung volumes by high-resolution computed tomography, St George's Respiratory Questionnaire, modified Medical Research Council dyspnea scale, and 6MWD. All adverse events were collected and independently adjudicated.
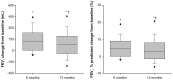
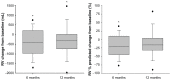
Results: Forty four patients were treated at a mean (standard deviation) age of 63 (5.6) years, FEV(1) 0.86 mL (0.25 mL) (n = 22 men and 22 women). Mean (standard deviation) changes from baseline at 12 months were: FEV(1) 86.2 mL (173.8 mL), St George's Respiratory Questionnaire -11.0 (14.0) units, treated lobar volume from high-resolution computed tomography -751.8 mL (653.9 mL), residual volume -302.8 mL (775.6 mL), 6MWD 18.5 m (63.7 m), and modified Medical Research Council dyspnea scale score -0.83 (0.97) (P < 0.05 for all except 6MWD). Improvements were numerically larger at 6 versus 12 months. GOLD stage III and IV patients had similar outcomes at 6 months; however, improvements relative to baseline were numerically higher in GOLD stage IV patients. Larger improvements were observed in patients with higher heterogeneity. In total, 39 serious adverse events were reported in 23 patients with 10 events in 8 patients between 6 and 12 months.
Conclusion: Unilateral lobar InterVapor treatment of heterogeneous emphysema improved lung function and health outcomes 1 year following treatment. The magnitude of improvement was larger at 6 months compared to 12 months. Improvements relative to baseline continue to be exhibited at 12 months despite the expected disease related decline over time.
Trial registration: ClinicalTrials.gov NCT01041586 NCT01102712.
Keywords: bronchoscopy; emphysema; lung volume reduction; thermal energy.
Figures

References
-
- Brantigan OC, Mueller E. Surgical treatment of pulmonary emphysema. Am Surg. 1957;23(9):789–804. - PubMed
-
- Cooper JD, Trulock EP, Triantafillou AN, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg. 1995;109(1):106–116. - PubMed
-
- The National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: A prospective randomized trial to lung volume reduction surgery. Chest. 1999;116(6):1750–1761. - PubMed
-
- The National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severed emphysema. N Engl J Med. 2003;348(21):2059–2073. - PubMed
-
- Naunheim KS, Wood DE, Mohsenifar Z, et al. National Emphysema Treatment Trial Research Group. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82:431–443. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

