Acetate activation in Methanosaeta thermophila: characterization of the key enzymes pyrophosphatase and acetyl-CoA synthetase
- PMID: 22927778
- PMCID: PMC3426162
- DOI: 10.1155/2012/315153
Acetate activation in Methanosaeta thermophila: characterization of the key enzymes pyrophosphatase and acetyl-CoA synthetase
Abstract
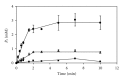
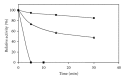
The thermophilic methanogen Methanosaeta thermophila uses acetate as sole substrate for methanogenesis. It was proposed that the acetate activation reaction that is needed to feed acetate into the methanogenic pathway requires the hydrolysis of two ATP, whereas the acetate activation reaction in Methanosarcina sp. is known to require only one ATP. As these organisms live at the thermodynamic limit that sustains life, the acetate activation reaction in Mt. thermophila seems too costly and was thus reevaluated. It was found that of the putative acetate activation enzymes one gene encoding an AMP-forming acetyl-CoA synthetase was highly expressed. The corresponding enzyme was purified and characterized in detail. It catalyzed the ATP-dependent formation of acetyl-CoA, AMP, and pyrophosphate (PP(i)) and was only moderately inhibited by PP(i). The breakdown of PP(i) was performed by a soluble pyrophosphatase. This enzyme was also purified and characterized. The pyrophosphatase hydrolyzed the major part of PP(i) (K(M) = 0.27 ± 0.05 mM) that was produced in the acetate activation reaction. Activity was not inhibited by nucleotides or PP(i). However, it cannot be excluded that other PP(i)-dependent enzymes take advantage of the remaining PP(i) and contribute to the energy balance of the cell.
Figures

References
-
- Khalil MAK, Rasmussen RA. Global emissions of methane during the last several centuries. Chemosphere. 1994;29(5):833–842.
-
- Conrad R, Klose M. Anaerobic conversion of carbon dioxide to methane, acetate and propionate on washed rice roots. FEMS Microbiology Ecology. 1999;30(2):147–155. - PubMed
-
- Reay DS. Sinking methane. Biologist. 2003;50(1):15–19. - PubMed
-
- Kashyap DR, Dadhich KS, Sharma SK. Biomethanation under psychrophilic conditions: a review. Bioresource Technology. 2003;87(2):147–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

