Evolutionarily repurposed networks reveal the well-known antifungal drug thiabendazole to be a novel vascular disrupting agent
- PMID: 22927795
- PMCID: PMC3423972
- DOI: 10.1371/journal.pbio.1001379
Evolutionarily repurposed networks reveal the well-known antifungal drug thiabendazole to be a novel vascular disrupting agent
Abstract
Studies in diverse organisms have revealed a surprising depth to the evolutionary conservation of genetic modules. For example, a systematic analysis of such conserved modules has recently shown that genes in yeast that maintain cell walls have been repurposed in vertebrates to regulate vein and artery growth. We reasoned that by analyzing this particular module, we might identify small molecules targeting the yeast pathway that also act as angiogenesis inhibitors suitable for chemotherapy. This insight led to the finding that thiabendazole, an orally available antifungal drug in clinical use for 40 years, also potently inhibits angiogenesis in animal models and in human cells. Moreover, in vivo time-lapse imaging revealed that thiabendazole reversibly disassembles newly established blood vessels, marking it as vascular disrupting agent (VDA) and thus as a potential complementary therapeutic for use in combination with current anti-angiogenic therapies. Importantly, we also show that thiabendazole slows tumor growth and decreases vascular density in preclinical fibrosarcoma xenografts. Thus, an exploration of the evolutionary repurposing of gene networks has led directly to the identification of a potential new therapeutic application for an inexpensive drug that is already approved for clinical use in humans.
Conflict of interest statement
A patent application based on this work has been filed. The authors have declared that no other competing interests exist.
Figures




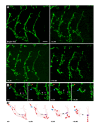
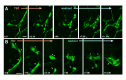
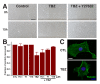
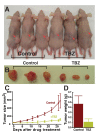

Comment in
-
Evolution-based drug discovery: antifungal also disrupts blood vessel formation.PLoS Biol. 2012;10(8):e1001380. doi: 10.1371/journal.pbio.1001380. Epub 2012 Aug 21. PLoS Biol. 2012. PMID: 22927796 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

