Prevalence and follow-up of occult HCV infection in an Italian population free of clinically detectable infectious liver disease
- PMID: 22927986
- PMCID: PMC3425488
- DOI: 10.1371/journal.pone.0043541
Prevalence and follow-up of occult HCV infection in an Italian population free of clinically detectable infectious liver disease
Abstract
Background: Occult hepatitis C virus infection (OCI) is a recently described phenomenon characterized by undetectable levels of HCV-RNA in serum/plasma by current laboratory assays, with identifiable levels in peripheral blood mononuclear cells (PBMCs) and/or liver tissue by molecular tests with enhanced sensitivity. Previous results from our group showed an OCI prevalence of 3.3% in a population unselected for hepatic disease. The present study aimed to evaluate OCI prevalence in a larger cohort of infectious liver disease-free (ILDF) subjects. Clinical follow-up of OCI subjects was performed to investigate the natural history of the infection.
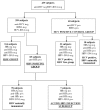
Methods and findings: 439 subjects referred to a Turin Blood Bank for phlebotomy therapy were recruited. They included 314 ILDF subjects, 40 HCV-positive subjects and 85 HBV-positive subjects, of whom 7 were active HBV carriers. Six subjects (4/314 ILDF subjects [1.27%] and 2/7 active HBV carriers [28%]) were positive for HCV-RNA in PBMCs, but negative for serological and virological markers of HCV, indicating OCI. HCV genotypes were determined in the PBMCs of 3/6 OCI subjects two had type 1b; the other had type 2a/2c. OCI subjects were followed up for at least 2 years. After 12 months only one OCI persisted, showing a low HCV viral load (3.73×10(1) UI/ml). By the end of follow-up all OCI subjects were negative for HCV. No seroconversion, alteration of liver enzyme levels, or reduction of liver synthesis occurred during follow-up.
Conclusions: This study demonstrated the existence of OCI in ILDF subjects, and suggested a high OCI prevalence among active HBV carriers. Follow-up suggested that OCI could be transient, with a trend toward the decrease of HCV viral load to levels undetectable by conventional methods after 12-18 months. Confirmation studies with a longer follow-up period are needed for identification of the OCI clearance or recurrence rates, and to characterize the viruses involved.
Conflict of interest statement
Figures
Similar articles
-
Prevalence of occult hepatitis C virus among hemodialysis patients in Tanta university hospitals: a single-center study.Environ Sci Pollut Res Int. 2018 Feb;25(6):5459-5464. doi: 10.1007/s11356-017-0897-y. Epub 2017 Dec 6. Environ Sci Pollut Res Int. 2018. PMID: 29214477
-
Occult Hepatitis C Virus Infection among Hemodialysis Patients: An Iranian Experience.Arch Iran Med. 2020 Sep 1;23(9):586-592. doi: 10.34172/aim.2020.68. Arch Iran Med. 2020. PMID: 32979904
-
Occurrence of occult HCV infection among Hiv infected patients in Georgia.Georgian Med News. 2014 Jan;(226):37-41. Georgian Med News. 2014. PMID: 24523330
-
Seronegative occult hepatitis C virus infection: clinical implications.J Clin Virol. 2014 Nov;61(3):315-20. doi: 10.1016/j.jcv.2014.09.007. Epub 2014 Sep 28. J Clin Virol. 2014. PMID: 25304062 Review.
-
Understanding occult hepatitis C infection.Transfusion. 2020 Sep;60(9):2144-2152. doi: 10.1111/trf.16006. Epub 2020 Aug 15. Transfusion. 2020. PMID: 33460181 Review.
Cited by
-
Occult Hepatitis C Virus Infection in Hemodialysis Patients; Single Center Study.Electron Physician. 2015 Dec 20;7(8):1619-25. doi: 10.19082/1619. eCollection 2015 Dec. Electron Physician. 2015. PMID: 26816589 Free PMC article.
-
Occult HCV Infection: The Current State of Knowledge.Iran Red Crescent Med J. 2015 Nov 29;17(11):e34181. doi: 10.5812/ircmj.34181. eCollection 2015 Nov. Iran Red Crescent Med J. 2015. PMID: 26734487 Free PMC article. Review.
-
Prevalence and Risk Factors of Occult HCV Infection in the Adult Population of Mexico City.Viruses. 2025 Feb 8;17(2):236. doi: 10.3390/v17020236. Viruses. 2025. PMID: 40006991 Free PMC article.
-
Occult hepatitis C virus infection in candidates for liver transplant with cryptogenic cirrhosis.Hepat Mon. 2013 Aug 5;13(8):e11290. doi: 10.5812/hepatmon.11290. eCollection 2013. Hepat Mon. 2013. PMID: 24082889 Free PMC article.
-
A New Twist to a Chronic HCV Infection: Occult Hepatitis C.Gastroenterol Res Pract. 2015;2015:579147. doi: 10.1155/2015/579147. Epub 2015 Jun 24. Gastroenterol Res Pract. 2015. PMID: 26221136 Free PMC article. Review.
References
-
- Castillo I, Pardo M, Bartolomè J, Ortiz-Movilla N, Rodriguez-Inigo E, et al. (2004) Occult hepatitis C virus infection in patients in whom the etiology of persistently abnormal results of liver-function tests is unknown. J Infect Dis 189: 7–14. - PubMed
-
- Welker MW, Zeuzem S (2009) Occult hepatitis C: how convincing are the current data? Hepatology 49: 665–675. - PubMed

