Ionizing radiation induces stemness in cancer cells
- PMID: 22928007
- PMCID: PMC3424153
- DOI: 10.1371/journal.pone.0043628
Ionizing radiation induces stemness in cancer cells
Erratum in
- PLoS One. 2013;8(3). doi: 10.1371/annotation/15587a16-d9c2-446b-9601-924c2db5c5d9 doi: 10.1371/annotation/15587a16-d9c2-446b-9601-924c2db5c5d9
Abstract
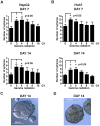
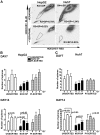
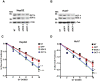
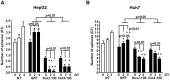
The cancer stem cell (CSC) model posits the presence of a small number of CSCs in the heterogeneous cancer cell population that are ultimately responsible for tumor initiation, as well as cancer recurrence and metastasis. CSCs have been isolated from a variety of human cancers and are able to generate a hierarchical and heterogeneous cancer cell population. CSCs are also resistant to conventional chemo- and radio-therapies. Here we report that ionizing radiation can induce stem cell-like properties in heterogeneous cancer cells. Exposure of non-stem cancer cells to ionizing radiation enhanced spherogenesis, and this was accompanied by upregulation of the pluripotency genes Sox2 and Oct3/4. Knockdown of Sox2 or Oct3/4 inhibited radiation-induced spherogenesis and increased cellular sensitivity to radiation. These data demonstrate that ionizing radiation can activate stemness pathways in heterogeneous cancer cells, resulting in the enrichment of a CSC subpopulation with higher resistance to radiotherapy.
Conflict of interest statement
Figures

References
-
- Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730–737. - PubMed
-
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, et al. (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, et al. (2004) Identification of human brain tumour initiating cells. Nature 432: 396–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

