Phaeobacter gallaeciensis reduces Vibrio anguillarum in cultures of microalgae and rotifers, and prevents vibriosis in cod larvae
- PMID: 22928051
- PMCID: PMC3425499
- DOI: 10.1371/journal.pone.0043996
Phaeobacter gallaeciensis reduces Vibrio anguillarum in cultures of microalgae and rotifers, and prevents vibriosis in cod larvae
Abstract
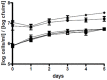
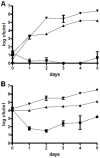
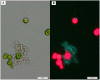
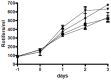
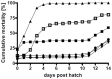
Phaeobacter gallaeciensis can antagonize fish-pathogenic bacteria in vitro, and the purpose of this study was to evaluate the organism as a probiont for marine fish larvae and their feed cultures. An in vivo mechanism of action of the antagonistic probiotic bacterium is suggested using a non-antagonistic mutant. P. gallaeciensis was readily established in axenic cultures of the two microalgae Tetraselmis suecica and Nannochloropsis oculata, and of the rotifer Brachionus plicatilis. P. gallaeciensis reached densities of 10(7) cfu/ml and did not adversely affect growth of algae or rotifers. Vibrio anguillarum was significantly reduced by wild-type P. gallaeciensis, when introduced into these cultures. A P. gallaeciensis mutant that did not produce the antibacterial compound tropodithietic acid (TDA) did not reduce V. anguillarum numbers, suggesting that production of the antibacterial compound is important for the antagonistic properties of P. gallaeciensis. The ability of P. gallaeciensis to protect fish larvae from vibriosis was determined in a bath challenge experiment using a multidish system with 1 larva per well. Unchallenged larvae reached 40% accumulated mortality which increased to 100% when infected with V. anguillarum. P. gallaeciensis reduced the mortality of challenged cod larvae (Gadus morhua) to 10%, significantly below the levels of both the challenged and the unchallenged larvae. The TDA mutant reduced mortality of the cod larvae in some of the replicates, although to a much lesser extent than the wild type. It is concluded that P. gallaeciensis is a promising probiont in marine larviculture and that TDA production likely contributes to its probiotic effect.
Conflict of interest statement
Figures


References
-
- Olafsen JA (2001) Interactions between fish larvae and bacteria in marine aquaculture. Aquacult 200: 223–247.
-
- Toranzo AE, Magarinos B, Romalde JL (2005) A review of the main bacterial fish diseases 3. in mariculture systems. Aquacult 246: 37–61.
-
- Wietz M, Gram L, Jorgensen B, Schramm A (2010) Latitudinal patterns in the abundance of major marine bacterioplankton groups. Aquat Microb Ecol 61: 179–189.
-
- Douillet PA, Pickering PL (1999) Seawater treatment for larval culture of the fish Sciaenops ocellatus Linnaeus (red drum). Aquacult 170: 113–126.
-
- Eddy SD, Jones SH (2002) Microbiology of summer flounder Paralichthys dentatus fingerling production at a marine fish hatchery. Aquacult 211: 9–28.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

