Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view
- PMID: 22928052
- PMCID: PMC3424254
- DOI: 10.1371/journal.pntd.0001763
Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view
Abstract
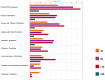
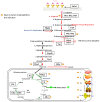
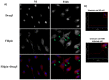
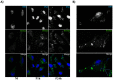
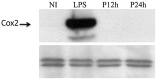
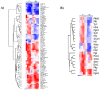
We analyzed the transcriptional signatures of mouse bone marrow-derived macrophages at different times after infection with promastigotes of the protozoan parasite Leishmania major. Ingenuity Pathway Analysis revealed that the macrophage metabolic pathways including carbohydrate and lipid metabolisms were among the most altered pathways at later time points of infection. Indeed, L. major promastiogtes induced increased mRNA levels of the glucose transporter and almost all of the genes associated with glycolysis and lactate dehydrogenase, suggesting a shift to anaerobic glycolysis. On the other hand, L. major promastigotes enhanced the expression of scavenger receptors involved in the uptake of Low-Density Lipoprotein (LDL), inhibited the expression of genes coding for proteins regulating cholesterol efflux, and induced the synthesis of triacylglycerides. These data suggested that Leishmania infection disturbs cholesterol and triglycerides homeostasis and may lead to cholesterol accumulation and foam cell formation. Using Filipin and Bodipy staining, we showed cholesterol and triglycerides accumulation in infected macrophages. Moreover, Bodipy-positive lipid droplets accumulated in close proximity to parasitophorous vacuoles, suggesting that intracellular L. major may take advantage of these organelles as high-energy substrate sources. While the effect of infection on cholesterol accumulation and lipid droplet formation was independent on parasite development, our data indicate that anaerobic glycolysis is actively induced by L. major during the establishment of infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, et al. (2003) Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102: 672–681. - PubMed
-
- Buates S, Matlashewski G (2001) General suppression of macrophage gene expression during Leishmania donovani infection. J Immunol 166: 3416–3422. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

