Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial
- PMID: 22928076
- PMCID: PMC3427199
- DOI: 10.4161/derm.20332
Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial
Abstract
<u>
Background: </u> Vitamin D insufficiency is common in cystic fibrosis (CF) and vitamin D repletion may have an important role in improving clinical outcomes in CF. This randomized, placebo-controlled, pilot study examined the feasibility and impact of a single, large dose of cholecalciferol on vitamin D status and clinical outcomes in subjects with CF. <u>
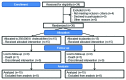
Methods: </u> Thirty adults with were randomized in a double-blinded, pilot study to receive 250,000 IU cholecalciferol or placebo within 48 h of hospital admission for a pulmonary exacerbation. Concentrations of 25-hydroxyvitamin D (25(OH)D), clinical outcomes and potential adverse events were assessed up to one year after randomization. Mixed effects linear regression models were used to evaluate the difference in mean serum concentrations and log-rank analyses were used to evaluate survival. <u>
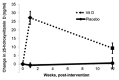
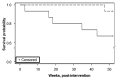
Results: </u> Data from all subjects was analyzed. Serum 25(OH)D concentrations increased from a mean of 30.6 ± 3.2 ng/mL to 58.1 ± 3.5 ng/mL (p < 0.001) at one week and 36.7 ± 2.6 ng/mL by 12 weeks (p = 0.06) in the vitamin D group; in contrast, serum 25(OH)D concentrations remained unchanged in the placebo group. Unadjusted, one-year survival and hospital-free days were increased in the vitamin D group (p = 0.029, p = 0.036; respectively). There was also a trend toward increased IV antibiotic therapy-free days in the vitamin D group (p = 0.073). There were no signs of hypervitaminosis D or adverse events. Serum PTH and calcium concentrations were similar across both groups. <u>
Conclusions: </u> In this pilot study, a single, oral bolus of cholecalciferol increased serum 25(OH)D concentrations and was associated with a trend toward improved clinical outcomes in CF subjects hospitalized for a pulmonary exacerbation. Further investigation is needed into the clinical impact of improved vitamin D status in patients with CF.
Keywords: anti-microbial peptide; cystic fibrosis; parathyroid hormone; pulmonary exacerbation; vitamin D.
Figures
References
-
- Foundation, Fibrosis C. Patient Registry 2009 Annual Report ed. Bethesda, MD, USA: Cystic Fibrosis Foundation 2010.
-
- Khazai NB, Judd SE, Jeng L, Wolfenden LL, Stecenko A, Ziegler TR, et al. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab. 2009;94:2037–43. doi: 10.1210/jc.2008-2012. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
