Cognitive deterioration and associated pathology induced by chronic low-level aluminum ingestion in a translational rat model provides an explanation of Alzheimer's disease, tests for susceptibility and avenues for treatment
- PMID: 22928148
- PMCID: PMC3423924
- DOI: 10.1155/2012/914947
Cognitive deterioration and associated pathology induced by chronic low-level aluminum ingestion in a translational rat model provides an explanation of Alzheimer's disease, tests for susceptibility and avenues for treatment
Abstract
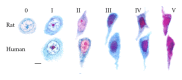
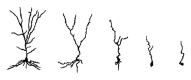
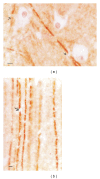
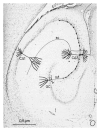
A translational aging rat model for chronic aluminum (Al) neurotoxicity mimics human Al exposure by ingesting Al, throughout middle age and old age, in equivalent amounts to those ingested by Americans from their food, water, and Al additives. Most rats that consumed Al in an amount equivalent to the high end of the human total dietary Al range developed severe cognitive deterioration in old age. High-stage Al accumulation occurred in the entorhinal cortical cells of origin for the perforant pathway and hippocampal CA1 cells, resulting in microtubule depletion and dendritic dieback. Analogous pathological change in humans leads to destruction of the perforant pathway and Alzheimer's disease dementia. The hippocampus is thereby isolated from neocortical input and output normally mediated by the entorhinal cortex. Additional evidence is presented that Al is involved in the formation of neurofibrillary tangles, amyloid plaques, granulovacuolar degeneration, and other pathological changes of Alzheimer's disease (AD). The shared characteristics indicate that AD is a human form of chronic Al neurotoxicity. This translational animal model provides fresh strategies for the prevention, diagnosis, and treatment of AD.
Figures




References
-
- Price M, Jackson J, editors. Alzheimer’s Disease International World Alzheimer Report 2009, http://www.alz.co.uk/research/files/World%20Alzheimer%20Report.pdf.
-
- Morris JC. Differential diagnosis of Alzheimer’s disease. Clinics in Geriatric Medicine. 1994;10(2):257–276. - PubMed
-
- Humphry GM. Old Age: The Results of Information Received Respecting Nearly Nine Hundred Persons Who Had Attained the Age of Eighty Years, Including Seventy-Four Centenarians. Cambridge, UK: MacMillan and Bowes; 1889.
-
- Henderson AS. The epidemiology of Alzheimer’s disease. British Medical Bulletin. 1986;42(1):3–10. - PubMed
-
- Blansjaar BA, Thomassen R, Van Schaick HW. Prevalence of dementia in centenarians. International Journal of Geriatric Psychiatry. 2000;15(3):219–225. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

