Fusion to a highly charged proteasomal retargeting sequence increases soluble cytoplasmic expression and efficacy of diverse anti-synuclein intrabodies
- PMID: 22929188
- PMCID: PMC3502235
- DOI: 10.4161/mabs.21696
Fusion to a highly charged proteasomal retargeting sequence increases soluble cytoplasmic expression and efficacy of diverse anti-synuclein intrabodies
Abstract
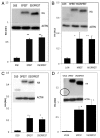
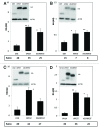
Intrabodies can be powerful reagents to effect modulation of aberrant intracellular proteins that underlie a range of diseases. However, their cytoplasmic solubility can be limiting. We previously reported that overall charge and hydrophilicity can be combined to provide initial estimates of intracellular solubility, and that charge engineering via fusion can alter solubility properties experimentally. Additional studies showed that fusion of a proteasome-targeting PEST motif to the anti-huntingtin intrabody scFv-C4 can degrade mutant huntingtin proteins by directing them to the proteasome, while also increasing the negative charge. We now validate the generality of this approach with intrabodies against α-synuclein (α-syn), an important target in Parkinson disease. In this study, fusion of the PEST sequence to a set of four diverse, poorly soluble anti-α-syn intrabodies (D5E, 10H, D10 scFv, VH14 nanobody) significantly increased steady-state soluble intrabody protein levels in all cases, despite fusion with the PEST proteasomal-targeting signal. Furthermore, adding this PEST motif to the least soluble construct, VH14, significantly enhanced degradation of the target protein, α-syn~GFP. The intrabody-PEST fusion approach thus has dual advantages of potentially solubilizing intrabodies and enhancing their functionality in parallel. Empirical testing of intrabody-PEST fusions is recommended for enhancement of intrabody solubility from diverse sources.
Keywords: Parkinson disease; intrabodies; intrabody-PEST fusions; proteasome; α-synuclein.
Figures

Similar articles
-
Bifunctional anti-huntingtin proteasome-directed intrabodies mediate efficient degradation of mutant huntingtin exon 1 protein fragments.PLoS One. 2011;6(12):e29199. doi: 10.1371/journal.pone.0029199. Epub 2011 Dec 22. PLoS One. 2011. PMID: 22216210 Free PMC article.
-
Solubility Characterization and Imaging of Intrabodies Using GFP-Fusions.Methods Mol Biol. 2017;1575:165-174. doi: 10.1007/978-1-4939-6857-2_9. Methods Mol Biol. 2017. PMID: 28255879
-
Physico-chemical determinants of soluble intrabody expression in mammalian cell cytoplasm.Protein Eng Des Sel. 2010 Jun;23(6):489-98. doi: 10.1093/protein/gzq022. Epub 2010 Apr 8. Protein Eng Des Sel. 2010. PMID: 20378699 Free PMC article.
-
Intrabody and Parkinson's disease.Biochim Biophys Acta. 2009 Jul;1792(7):634-42. doi: 10.1016/j.bbadis.2008.09.001. Epub 2008 Sep 12. Biochim Biophys Acta. 2009. PMID: 18834937 Free PMC article. Review.
-
Intracellular antibodies (intrabodies) and their therapeutic potential.Handb Exp Pharmacol. 2008;(181):343-73. doi: 10.1007/978-3-540-73259-4_15. Handb Exp Pharmacol. 2008. PMID: 18071953 Review.
Cited by
-
Treating Parkinson's Disease with Antibodies: Previous Studies and Future Directions.J Parkinsons Dis. 2021;11(1):71-92. doi: 10.3233/JPD-202221. J Parkinsons Dis. 2021. PMID: 33104039 Free PMC article. Review.
-
Alpha-Synuclein Targeting Therapeutics for Parkinson's Disease and Related Synucleinopathies.Front Neurol. 2022 May 9;13:852003. doi: 10.3389/fneur.2022.852003. eCollection 2022. Front Neurol. 2022. PMID: 35614915 Free PMC article. Review.
-
Modifications of a signal sequence for antibody secretion from insect cells.Cytotechnology. 2018 Jun;70(3):891-898. doi: 10.1007/s10616-017-0109-0. Epub 2017 Jun 5. Cytotechnology. 2018. PMID: 28584932 Free PMC article.
-
Immunotherapeutic Approaches Targeting Amyloid-β, α-Synuclein, and Tau for the Treatment of Neurodegenerative Disorders.Neurotherapeutics. 2016 Jan;13(1):179-89. doi: 10.1007/s13311-015-0397-z. Neurotherapeutics. 2016. PMID: 26494242 Free PMC article. Review.
-
Specific in vivo knockdown of protein function by intrabodies.MAbs. 2015;7(6):1010-35. doi: 10.1080/19420862.2015.1076601. Epub 2015 Aug 7. MAbs. 2015. PMID: 26252565 Free PMC article. Review.
References
-
- Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotný J, Margolies MN, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988;85:5879–83. doi: 10.1073/pnas.85.16.5879. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
