ROCK-phosphorylated vimentin modifies mutant huntingtin aggregation via sequestration of IRBIT
- PMID: 22929228
- PMCID: PMC3502191
- DOI: 10.1186/1750-1326-7-43
ROCK-phosphorylated vimentin modifies mutant huntingtin aggregation via sequestration of IRBIT
Abstract
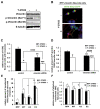
Background: Huntington's Disease (HD) is a fatal hereditary neurodegenerative disease caused by the accumulation of mutant huntingtin protein (Htt) containing an expanded polyglutamine (polyQ) tract. Activation of the channel responsible for the inositol-induced Ca²⁺ release from ensoplasmic reticulum (ER), was found to contribute substantially to neurodegeneration in HD. Importantly, chemical and genetic inhibition of inositol 1,4,5-trisphosphate (IP3) receptor type 1 (IP3R1) has been shown to reduce mutant Htt aggregation.
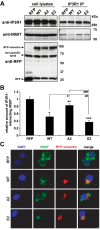
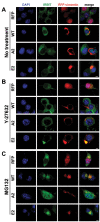
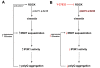
Results: In this study, we propose a novel regulatory mechanism of IP3R1 activity by type III intermediate filament vimentin which sequesters the negative regulator of IP3R1, IRBIT, into perinuclear inclusions, and reduces its interaction with IP3R1 resulting in promotion of mutant Htt aggregation. Proteasome inhibitor MG132, which causes polyQ proteins accumulation and aggregation, enhanced the sequestration of IRBIT. Furthermore we found that IRBIT sequestration can be prevented by a rho kinase inhibitor, Y-27632.
Conclusions: Our results suggest that vimentin represents a novel and additional target for the therapy of polyQ diseases.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

