The peptide derived from the Ig-like domain of human herpesvirus 8 K1 protein induces death in hematological cancer cells
- PMID: 22929310
- PMCID: PMC3517441
- DOI: 10.1186/1756-9966-31-69
The peptide derived from the Ig-like domain of human herpesvirus 8 K1 protein induces death in hematological cancer cells
Abstract
Background: Although significant progress has been made in the treatment of lymphomas, many lymphomas exhibit resistance to cell death, suggesting a defective Fas signaling, which remains poorly understood. We previously reported that cells expressing the K1 protein of human herpesvirus 8 (HHV-8) resist death through the complex formation of the Ig-like domain of K1 with Fas. Recently, we investigated whether peptides derived from the Ig-like domain of the K1 protein may affect cell death.
Methods: K1 positive and negative cell lines were incubated with the K1-derived peptides, and cell death (apoptotic and necrotic) was assessed by flow cytometry and LDH assay. Activation of caspases was assessed by fluorometric assay and flow cytometry. Fas receptor-independent, peptide-mediated cell killing was tested in the Fas-resistant Daudi cell line and Jurkat cell clones deficient in caspase-8 and FADD functionality. Activation of TNF receptors I and II was blocked by pre-incubation with corresponding blocking antibodies. The effect of the K1 peptide in vivo was tested in a mouse xenograft model.
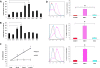
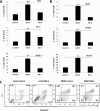
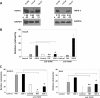
Results: We observed that the peptide S20-3 enhanced cell death in K1-positive BJAB cells and HHV-8 positive primary effusion lymphoma (PEL) cell lines. Similar effects of this peptide were observed in B-cell lymphoma and T-lymphoblastic leukemia cells without K1 expression but not in normal human peripheral blood mononuclear cells. A single intratumoral injection of the S20-3 peptide decreased the growth of Jurkat xenografts in SCID mice. The mechanism of tumor cell death induced by the S20-3 peptide was associated with activation of caspases, but this activity was only partially inhibited by the pan-caspase inhibitor z-VAD. Furthermore, the K1 peptide also killed Fas-resistant Daudi cells, and this killing effect was inhibited by pre-incubation of cells with antibodies blocking TNFRI.
Conclusion: Taken together, these findings indicate that the S20-3 peptide can selectively induce the death of malignant hematological cell lines by Fas- and/or TNFRI-dependent mechanisms, suggesting the K1-derived peptide or peptidomimetic may have promising therapeutic potential for the treatment of hematological cancers.
Figures


References
-
- Muller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH, Galle PR. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Invest. 1997;99:403–413. doi: 10.1172/JCI119174. - DOI - PMC - PubMed
-
- Vega MI, Huerta-Yepez S, Jazirehi AR, Garban H, Bonavida B. Rituximab (chimeric anti-CD20) sensitizes B-NHL cell lines to Fas-induced apoptosis. Oncogene. 2005;24:8114–8127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

