Drug transport across the blood-brain barrier
- PMID: 22929442
- PMCID: PMC3494002
- DOI: 10.1038/jcbfm.2012.126
Drug transport across the blood-brain barrier
Abstract
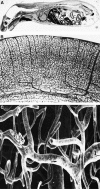
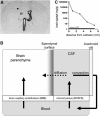
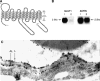
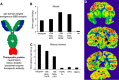
The blood-brain barrier (BBB) prevents the brain uptake of most pharmaceuticals. This property arises from the epithelial-like tight junctions within the brain capillary endothelium. The BBB is anatomically and functionally distinct from the blood-cerebrospinal fluid barrier at the choroid plexus. Certain small molecule drugs may cross the BBB via lipid-mediated free diffusion, providing the drug has a molecular weight <400 Da and forms <8 hydrogen bonds. These chemical properties are lacking in the majority of small molecule drugs, and all large molecule drugs. Nevertheless, drugs can be reengineered for BBB transport, based on the knowledge of the endogenous transport systems within the BBB. Small molecule drugs can be synthesized that access carrier-mediated transport (CMT) systems within the BBB. Large molecule drugs can be reengineered with molecular Trojan horse delivery systems to access receptor-mediated transport (RMT) systems within the BBB. Peptide and antisense radiopharmaceuticals are made brain-penetrating with the combined use of RMT-based delivery systems and avidin-biotin technology. Knowledge on the endogenous CMT and RMT systems expressed at the BBB enable new solutions to the problem of BBB drug transport.
Figures


Comment in
-
Accessing the brain: the nose may know the way.J Cereb Blood Flow Metab. 2013 May;33(5):793-4. doi: 10.1038/jcbfm.2013.41. Epub 2013 Mar 13. J Cereb Blood Flow Metab. 2013. PMID: 23486291 Free PMC article. No abstract available.
References
-
- Reiber H, Felgenhauer K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta. 1987;163:319–328. - PubMed
-
- Friedemann U. Blood-brain barrier. Physiol Rev. 1942;22:125–145.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

