Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome
- PMID: 22929468
- PMCID: PMC3743220
- DOI: 10.1097/HP.0b013e3182666d6f
Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome
Abstract
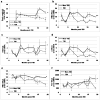
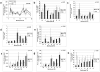
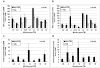
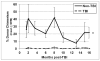
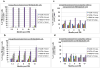
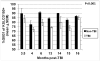
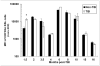
Residual bone marrow damage (RBMD) persists for years following exposure to radiation and is believed to be due to decreased self-renewal potential of radiation-damaged hematopoietic stem cells (HSC). Current literature has examined primarily sublethal doses of radiation and time points within a few months of exposure. In this study, the authors examined RBMD in mice surviving lethal doses of total body ionizing irradiation (TBI) in a murine model of the Hematopoietic Syndrome of the Acute Radiation Syndrome (H-ARS). Survivors were analyzed at various time points up to 19 mo post-TBI for hematopoietic function. The competitive bone marrow (BM) repopulating potential of 150 purified c-Kit+ Sca-1+ lineage- CD150+ cells (KSLCD150+) remained severely deficient throughout the study compared to KSLCD150+ cells from non-TBI age-matched controls. The minimal engraftment from these TBI HSCs is predominantly myeloid, with minimal production of lymphocytes both in vitro and in vivo. All classes of blood cells as well as BM cellularity were significantly decreased in TBI mice, especially at later time points as mice aged. Primitive BM hematopoietic cells (KSLCD150+) displayed significantly increased cell cycling in TBI mice at all time points, which may be a physiological attempt to maintain HSC numbers in the post-irradiation state. Taken together, these data suggest that the increased cycling among primitive hematopoietic cells in survivors of lethal radiation may contribute to long-term HSC exhaustion and subsequent RBMD, exacerbated by the added insult of aging at later time points.
Conflict of interest statement
No financial conflict of interest was declared by any of the authors.
Figures
References
-
- Belkacemi Y, Bouchet S, Frick J, Huchet A, Pene F, Aigueperse J, Gourmelon P, Lopez M, Gorin NC. Monitoring of residual hematopoiesis after total body irradiation in humans as a model for accidental x-ray exposure: dose-effect and failure of ex vivo expansion of residual stem cells in view of autografting. Int J Radiat Oncol Biol Phys. 2003;57:500–7. - PubMed
-
- Boggs D. The total marrow mass of the mouse: A simplified method of measurement. Am J Hematol. 1984;16:277–286. - PubMed
-
- Botnick LE, Hannon EC, Hellman S. A long lasting proliferative defect in the hematopoietic stem cell compartment following cytotoxic agents. Int J Radiat Oncol Biol Phys. 1979;5:1621–5. - PubMed
-
- Carsten A, Bond V. CFU content of the x-ray exposed and shielded mouse femur. Exptl Hematology. 1968;15:95–103.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

