The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation
- PMID: 22929471
- PMCID: PMC4140097
- DOI: 10.1097/HP.0b013e318266eb4c
The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation
Abstract
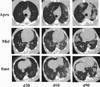
The dose response relationship for the acute gastrointestinal syndrome following total-body irradiation prevents analysis of the full recovery and damage to the gastrointestinal system, since all animals succumb to the subsequent 100% lethal hematopoietic syndrome. A partial-body irradiation model with 5% bone marrow sparing was established to investigate the prolonged effects of high-dose radiation on the gastrointestinal system, as well as the concomitant hematopoietic syndrome and other multi-organ injury including the lung. Herein, cellular and clinical parameters link acute and delayed coincident sequelae to radiation dose and time course post-exposure. Male rhesus Macaca mulatta were exposed to partial-body irradiation with 5% bone marrow (tibiae, ankles, feet) sparing using 6 MV linear accelerator photons at a dose rate of 0.80 Gy min(-1) to midline tissue (thorax) doses in the exposure range of 9.0 to 12.5 Gy. Following irradiation, all animals were monitored for multiple organ-specific parameters for 180 d. Animals were administered medical management including administration of intravenous fluids, antiemetics, prophylactic antibiotics, blood transfusions, antidiarrheals, supplemental nutrition, and analgesics. The primary endpoint was survival at 15, 60, or 180 d post-exposure. Secondary endpoints included evaluation of dehydration, diarrhea, hematologic parameters, respiratory distress, histology of small and large intestine, lung radiographs, and mean survival time of decedents. Dose- and time-dependent mortality defined several organ-specific sequelae, with LD50/15 of 11.95 Gy, LD50/60 of 11.01 Gy, and LD50/180 of 9.73 Gy for respective acute gastrointestinal, combined hematopoietic and gastrointestinal, and multi-organ delayed injury to include the lung. This model allows analysis of concomitant multi-organ sequelae, thus providing a link between acute and delayed radiation effects. Specific and multi-organ medical countermeasures can be assessed for efficacy and interaction during the concomitant evolution of acute and delayed key organ-specific subsyndromes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






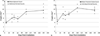







References
-
- Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing radiation lethality. Health Phys. 2003;84:565–575. - PubMed
-
- Bagdasarov AA, Raushenbakk MO, Abdullaev GM, Beliaeva BF, Lagutina NY. The treatment of acute radiation sickness with packed platelets. Probl Hematol Blood Transfus. 1959;4:1–5. - PubMed
-
- Baltschukat K, Nothdurft W. Hematological effects of unilateral and bilateral exposures of dogs to 300-kVp x rays. Radiat Res. 1990;123:7–16. - PubMed
-
- Baranov AE. Allogenic bone marrow transplantation after severe, uniform total-body irradiation: experience from recent (Nyasvizh, Belarus) and previous radiation accidents. In: MacVittie TJ, Weiss JF, Browne D, editors. Advances in the treatment of radiation injuries: advances in the biosciences. Vol. 94. Tarrytown, NY: Pergamon; Elsevier Science Ltd; 1996. pp. 281–293.
-
- Baranov AE, Guskova AK. Acute radiation disease in Chernobyl accident victims. In: Ricks RC, Fry SA, editors. The medical basis for radiation accident preparedness II; clinical experience and follow-up since 1979. New York: Elsevier; 1990. pp. 79–87.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

