Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial
- PMID: 22930695
- PMCID: PMC3533384
- DOI: 10.1136/bjsports-2012-091115
Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial
Abstract
Objectives: To assess if continuous topical glyceryl trinitrate (GTN) treatment improves outcome in patients with chronic patellar tendinopathy when compared with eccentric training alone.
Methods: Randomised double-blind, placebo-controlled clinical trial comparing a 12-week programme of using a GTN or placebo patch in combination with eccentric squats on a decline board. Measurements were performed at baseline, 6, 12 and 24 weeks. Primary outcome measure was the Victorian Institute of Sports Assessment-Patella (VISA-P) questionnaire. Secondary outcome measures were patient satisfaction and pain scores during sports. Generalised estimated equation was used to analyse the treatment, time and treatment×time effect. Analyses were performed following the intention-to-treat principle.
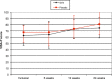
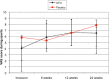
Results: VISA-P scores for both groups improved over the study period to 75.0±16.2 and 80.7±22.1 at 24 weeks. Results showed a significant effect for time (p<0.01) but no effect for treatment×time (p=0.80). Mean Visual Analogue Scores pain scores during sports for both groups increased over the study period to 6.6±3 and 7.8±3.1. Results showed a significant effect for time (p<0.01) but no effect for treatment×time (p=0.38). Patient satisfaction showed no difference between GTN and placebo groups (p=0.25) after 24 weeks, but did show a significant difference over time (p=0.01). Three patients in the GTN group reported some rash.
Conclusion: It seems that continuous topical GTN treatment in addition to an eccentric exercise programme does not improve clinical outcome compared to placebo patches and an eccentric exercise programme in patients with chronic patellar tendinopathy.
Figures

References
-
- Blazina ME, Kerlan RK, Jobe FW, et al. Jumper's knee. Orthop Clin North Am 1973;4:665–78 - PubMed
-
- Kannus P. Etiology and pathophysiology of chronic tendon disorders in sports. Scand J Med Sci Sports 1997;7:78–85 - PubMed
-
- Lian OB, Engebretsen L, Bahr R. Prevalence of jumper's knee among elite athletes from different sports —a cross-sectional study. Am J Sports Med 2005;33:561–7 - PubMed
-
- Zwerver J, Bredeweg SW, van den Akker-Scheek I. Prevalence of jumper's knee among nonelite athletes from different sports: a cross-sectional survey. Am J Sports Med 2011;39:1984–8 - PubMed
-
- Kettunen JA, Kvist M, Alanen E, et al. Long-term prognosis for jumper's knee in male athletes—a prospective follow-up study. Am J Sports Med 2002;30:689–92 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
