The contribution of hydrophobic residues in the pore-forming region of the ryanodine receptor channel to block by large tetraalkylammonium cations and Shaker B inactivation peptides
- PMID: 22930804
- PMCID: PMC3434103
- DOI: 10.1085/jgp.201210851
The contribution of hydrophobic residues in the pore-forming region of the ryanodine receptor channel to block by large tetraalkylammonium cations and Shaker B inactivation peptides
Abstract
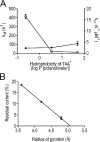
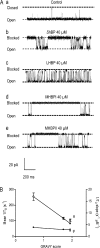
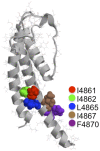
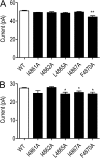
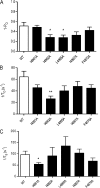
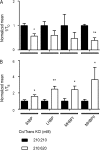
Although no high-resolution structural information is available for the ryanodine receptor (RyR) channel pore-forming region (PFR), molecular modeling has revealed broad structural similarities between this region and the equivalent region of K(+) channels. This study predicts that, as is the case in K(+) channels, RyR has a cytosolic vestibule lined with predominantly hydrophobic residues of transmembrane helices (TM10). In K(+) channels, this vestibule is the binding site for blocking tetraalkylammonium (TAA) cations and Shaker B inactivation peptides (ShBPs), which are stabilized by hydrophobic interactions involving specific residues of the lining helices. We have tested the hypothesis that the cytosolic vestibule of RyR fulfils a similar role and that TAAs and ShBPs are stabilized by hydrophobic interactions with residues of TM10. Both TAAs and ShBPs block RyR from the cytosolic side of the channel. By varying the composition of TAAs and ShBPs, we demonstrate that the affinity of both species is determined by their hydrophobicity, with variations reflecting alterations in the dissociation rate of the bound blockers. We investigated the role of TM10 residues of RyR by monitoring block by TAAs and ShBPs in channels in which the hydrophobicity of individual TM10 residues was lowered by alanine substitution. Although substitutions changed the kinetics of TAA interaction, they produced no significant changes in ShBP kinetics, indicating the absence of specific hydrophobic sites of interactions between RyR and these peptides. Our investigations (a) provide significant new information on both the mechanisms and structural components of the RyR PFR involved in block by TAAs and ShBPs, (b) highlight important differences in the mechanisms and structures determining TAA and ShBP block in RyR and K(+) channels, and (c) demonstrate that although the PFRs of these channels contain analogous structural components, significant differences in structure determine the distinct ion-handling properties of the two species of channel.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

