Dynamics of the L-fucose/H+ symporter revealed by fluorescence spectroscopy
- PMID: 22930818
- PMCID: PMC3443143
- DOI: 10.1073/pnas.1213445109
Dynamics of the L-fucose/H+ symporter revealed by fluorescence spectroscopy
Abstract
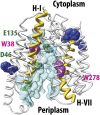
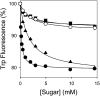
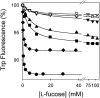
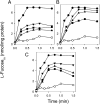
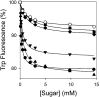
FucP of Escherichia coli catalyzes L-fucose/H(+) symport, and a crystal structure in an outward-facing conformation has been reported. However, nothing is known about FucP conformational dynamics. Here, we show that addition of L-fucose to purified FucP in detergent induces ∼20% quenching of Trp fluorescence in a concentration-dependent manner without a shift in λ(max). Quenching is essentially abolished when both Trp38 and Trp278, which are positioned on opposing faces of the outward-facing cavity walls, are replaced with Tyr or Phe, and reduced quenching is observed when either Trp is mutated. Therefore, both Trp residues are involved in the phenomenon. Furthermore, replacement of either Trp38 or Trp278, predominantly Trp38, causes decreased quenching, decreased apparent affinity for L-fucose, and significant inhibition of active L-fucose transport, indicating that the two residues are likely involved directly in sugar binding. It is proposed that sugar binding induces a conformational change in which the outward-facing cavity in FucP closes, thereby bringing Trp38 and Trp278 into close proximity around the bound sugar to form an "occluded" intermediate. The location of these two Trp residues provides a unique method for analyzing structural dynamics in FucP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Structure of a fucose transporter in an outward-open conformation.Nature. 2010 Oct 7;467(7316):734-8. doi: 10.1038/nature09406. Epub 2010 Sep 26. Nature. 2010. PMID: 20877283
-
Identification of a novel sugar-H+ symport protein, FucP, for transport of L-fucose into Escherichia coli.Mol Microbiol. 1994 Jun;12(5):799-809. doi: 10.1111/j.1365-2958.1994.tb01066.x. Mol Microbiol. 1994. PMID: 8052131
-
Probing of the rates of alternating access in LacY with Trp fluorescence.Proc Natl Acad Sci U S A. 2009 Dec 22;106(51):21561-6. doi: 10.1073/pnas.0911434106. Epub 2009 Dec 3. Proc Natl Acad Sci U S A. 2009. PMID: 19959662 Free PMC article.
-
The alternating access transport mechanism in LacY.J Membr Biol. 2011 Jan;239(1-2):85-93. doi: 10.1007/s00232-010-9327-5. Epub 2010 Dec 16. J Membr Biol. 2011. PMID: 21161516 Free PMC article. Review.
-
The lactose permease of Escherichia coli: overall structure, the sugar-binding site and the alternating access model for transport.FEBS Lett. 2003 Nov 27;555(1):96-101. doi: 10.1016/s0014-5793(03)01087-1. FEBS Lett. 2003. PMID: 14630326 Review.
Cited by
-
Evolutionary mix-and-match with MFS transporters.Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):5870-4. doi: 10.1073/pnas.1303538110. Epub 2013 Mar 25. Proc Natl Acad Sci U S A. 2013. PMID: 23530251 Free PMC article.
-
pH Regulation of Electrogenic Sugar/H+ Symport in MFS Sugar Permeases.PLoS One. 2016 May 26;11(5):e0156392. doi: 10.1371/journal.pone.0156392. eCollection 2016. PLoS One. 2016. PMID: 27227677 Free PMC article.
-
Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation.Proc Natl Acad Sci U S A. 2013 May 28;110(22):8876-81. doi: 10.1073/pnas.1306849110. Epub 2013 May 13. Proc Natl Acad Sci U S A. 2013. PMID: 23671103 Free PMC article.
-
Protonation of Glu(135) Facilitates the Outward-to-Inward Structural Transition of Fucose Transporter.Biophys J. 2015 Aug 4;109(3):542-51. doi: 10.1016/j.bpj.2015.06.037. Biophys J. 2015. PMID: 26244736 Free PMC article.
-
Functional architecture of MFS D-glucose transporters.Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):E719-27. doi: 10.1073/pnas.1400336111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24550316 Free PMC article.
References
-
- Becker DJ, Lowe JB. Fucose: Biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. - PubMed
-
- Staudacher E, Altmann F, Wilson IB, März L. Fucose in N-glycans: From plant to man. Biochim Biophys Acta. 1999;1473:216–236. - PubMed
-
- Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16:158R–184R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

