Ex vivo reconstitution of arterial endothelium by embryonic stem cell-derived endothelial progenitor cells in baboons
- PMID: 22931470
- PMCID: PMC3564485
- DOI: 10.1089/scd.2012.0313
Ex vivo reconstitution of arterial endothelium by embryonic stem cell-derived endothelial progenitor cells in baboons
Abstract
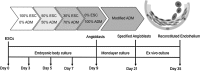
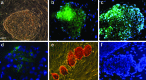
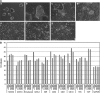
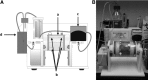
There is an increasing need for an animal model that can be used to translate basic research into clinical therapy. We documented the differentiation and functional competence of embryonic stem cell (ESC)-derived endothelial cells in baboons. Baboon angioblasts were sequentially differentiated from embryoid body cultures for 9 days in an angioblast differentiation medium with varying concentrations of BMP-4, FLT-3 ligand, stem cell factor, thrombopoietin, basic fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and knockout serum replacement. Real-time polymerase chain reaction results showed that ESC-derived angioblasts downregulated NANOG and OCT3/4, upregulated T-brachyury and GATA2, and moderately expressed CD34; they did not express CD144, TEK, or VWF, and varied in levels of CD31 expression. Several populations of putative angioblasts appeared 3 days and 9 days after differentiation, as identified by flow cytometry. Angioblasts at this stage exhibited dual paths of differentiation toward hematopoietic and vascular fates. To examine whether derived angioblasts could reconstitute the endothelium, we built an ex vivo culture system and seeded fluorescently labeled angioblast cultures onto a denuded segment of the femoral artery. We found that the seeded cells were able to grow into the endothelium on the interior surface of denuded artery segments within 5 days after seeding. After 14 days of ex vivo culture, the transplanted cells expressed CD31, an endothelial marker. The control arteries, seeded with vehicle only, did not harbor cells with endothelial markers. We conclude that ESC-derived angioblasts are promising therapeutic agents for repairing damaged vasculature, and that the baboon model will be vital for optimizing therapies for human clinical studies.
Figures




Similar articles
-
Endothelial reconstitution by CD34+ progenitors derived from baboon embryonic stem cells.J Cell Mol Med. 2013 Feb;17(2):242-51. doi: 10.1111/jcmm.12002. Epub 2013 Jan 10. J Cell Mol Med. 2013. PMID: 23301772 Free PMC article.
-
Efficient differentiation of human embryonic stem cells to arterial and venous endothelial cells under feeder- and serum-free conditions.Stem Cell Res Ther. 2015 Dec 30;6:261. doi: 10.1186/s13287-015-0260-5. Stem Cell Res Ther. 2015. PMID: 26718617 Free PMC article.
-
Canine retinal angioblasts are multipotent.Exp Eye Res. 2006 Jul;83(1):183-93. doi: 10.1016/j.exer.2005.09.025. Epub 2006 Mar 20. Exp Eye Res. 2006. PMID: 16545371
-
The role of FGF and VEGF in angioblast induction and migration during vascular development.Dev Dyn. 2001 Jan;220(1):1-17. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1087>3.0.CO;2-2. Dev Dyn. 2001. PMID: 11146503 Review.
-
Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance.J Cell Mol Med. 2004 Oct-Dec;8(4):498-508. doi: 10.1111/j.1582-4934.2004.tb00474.x. J Cell Mol Med. 2004. PMID: 15601578 Free PMC article. Review.
Cited by
-
Co-growth of Stem Cells With Target Tissue Culture as an Easy and Effective Method of Directed Differentiation.Front Bioeng Biotechnol. 2021 Jun 16;9:591775. doi: 10.3389/fbioe.2021.591775. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34222206 Free PMC article.
-
Personalized tissue-engineered arteries as vascular graft transplants: A safety study in sheep.Regen Ther. 2022 Sep 7;21:331-341. doi: 10.1016/j.reth.2022.08.005. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36110971 Free PMC article.
-
Vessel graft fabricated by the on-site differentiation of human mesenchymal stem cells towards vascular cells on vascular extracellular matrix scaffold under mechanical stimulation in a rotary bioreactor.J Mater Chem B. 2019 Apr 28;7(16):2703-2713. doi: 10.1039/c8tb03348j. Epub 2019 Mar 26. J Mater Chem B. 2019. PMID: 32255003 Free PMC article.
-
Tissue Engineered Small Vessel Conduits - The Anti-Thrombotic Effect of Re-Endothelialization of Decellularized Baboon Arteries: A Preliminary Experimental Study.Med Sci Monit Basic Res. 2017 Oct 30;23:344-351. doi: 10.12659/msmbr.905978. Med Sci Monit Basic Res. 2017. PMID: 29081492 Free PMC article.
-
Endothelial reconstitution by CD34+ progenitors derived from baboon embryonic stem cells.J Cell Mol Med. 2013 Feb;17(2):242-51. doi: 10.1111/jcmm.12002. Epub 2013 Jan 10. J Cell Mol Med. 2013. PMID: 23301772 Free PMC article.
References
-
- Abbott JD. Giordano FJ. Stem cells and cardiovascular disease. J Nucl Cardiol. 2003;10:403–412. - PubMed
-
- Ballard VL. Edelberg JM. Stem cells and the regeneration of the aging cardiovascular system. Circ Res. 2007;100:1116–1127. - PubMed
-
- Mitka M. Statin therapy in primary CVD prevention remains a hot-button topic for some. JAMA. 2011;306:2077–2078. - PubMed
-
- Libby P. Ridker PM. Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
