Stem cell factor gene transfer promotes cardiac repair after myocardial infarction via in situ recruitment and expansion of c-kit+ cells
- PMID: 22931954
- PMCID: PMC3651889
- DOI: 10.1161/CIRCRESAHA.111.263830
Stem cell factor gene transfer promotes cardiac repair after myocardial infarction via in situ recruitment and expansion of c-kit+ cells
Abstract
Rationale: There is growing evidence that the myocardium responds to injury by recruiting c-kit(+) cardiac progenitor cells to the damage tissue. Even though the ability of exogenously introducing c-kit(+) cells to injured myocardium has been established, the capability of recruiting these cells through modulation of local signaling pathways by gene transfer has not been tested.
Objective: To determine whether stem cell factor gene transfer mediates cardiac regeneration in a rat myocardial infarction model, through survival and recruitment of c-kit(+) progenitors and cell-cycle activation in cardiomyocytes, and explore the mechanisms involved.
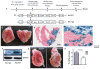
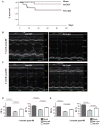
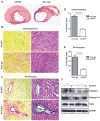
Methods and results: Infarct size, cardiac function, cardiac progenitor cells recruitment, fibrosis, and cardiomyocyte cell-cycle activation were measured at different time points in controls (n=10) and upon stem cell factor gene transfer (n=13) after myocardial infarction. We found a regenerative response because of stem cell factor overexpression characterized by an enhancement in cardiac hemodynamic function: an improvement in survival; a reduction in fibrosis, infarct size and apoptosis; an increase in cardiac c-kit(+) progenitor cells recruitment to the injured area; an increase in cardiomyocyte cell-cycle activation; and Wnt/β-catenin pathway induction.
Conclusions: Stem cell factor gene transfer induces c-kit(+) stem/progenitor cell expansion in situ and cardiomyocyte proliferation, which may represent a new therapeutic strategy to reverse adverse remodeling after myocardial infarction.
Figures


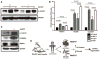
Comment in
-
Stem cell factor: a new weapon to regenerate the infarcted heart?Regen Med. 2012 Nov;7(6):754-5. Regen Med. 2012. PMID: 23304724 No abstract available.
References
-
- Lutz M, Rosenberg M, Kiessling F, Eckstein V, Heger T, Krebs J, Ho AD, Katus HA, Frey N. Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit + bone marrow-derived stem cells. Cardiovasc Res. 2008;77:143–150. - PubMed
-
- Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. - PubMed
-
- Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H, Zhang J, Cukerman E, Stanford WL, Medin JA, Liu PP. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:2304–2309. - PMC - PubMed
-
- Smith MA, Pallister CJ, Smith JG. Stem cell factor: biology and relevance to clinical practice. Acta Haematol. 2001;105:143–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL088434/HL/NHLBI NIH HHS/United States
- HHSN268201000045C/HL/NHLBI NIH HHS/United States
- P50 HL112324/HL/NHLBI NIH HHS/United States
- R01 HL088434/HL/NHLBI NIH HHS/United States
- P20HL100396/HL/NHLBI NIH HHS/United States
- R01 HL080498/HL/NHLBI NIH HHS/United States
- HL080498/HL/NHLBI NIH HHS/United States
- P20 HL100396/HL/NHLBI NIH HHS/United States
- HL083156/HL/NHLBI NIH HHS/United States
- R01 HL083156/HL/NHLBI NIH HHS/United States
- R01 HL093183/HL/NHLBI NIH HHS/United States
- T32 HL007824/HL/NHLBI NIH HHS/United States
- HL071753/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

