IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival
- PMID: 22932670
- PMCID: PMC3500434
- DOI: 10.1158/1078-0432.CCR-12-2130
IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival
Abstract
Purpose: Glioblastoma multiforme (GBM) is an aggressive adult brain tumor with a poor prognosis. One hallmark of GBM is the accumulation of immunosuppressive and tumor-promoting CD4(+)FoxP3(+)GITR(+) regulatory T cells (Tregs). Here, we investigated the role of indoleamine 2,3 dioxygenase (IDO) in brain tumors and the impact on Treg recruitment.
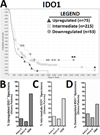
Experimental design: To determine the clinical relevance of IDO expression in brain tumors, we first correlated patient survival to the level of IDO expression from resected glioma specimens. We also used novel orthotopic and transgenic models of glioma to study how IDO affects Tregs. The impact of tumor-derived and peripheral IDO expression on Treg recruitment, GITR expression, and long-term survival was determined.
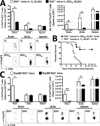
Results: Downregulated IDO expression in glioma predicted a significantly better prognosis in patients. Coincidently, both IDO-competent and deficient mice showed a survival advantage bearing IDO-deficient brain tumors, when compared with IDO-competent brain tumors. Moreover, IDO deficiency was associated with a significant decrease in brain-resident Tregs, both in orthotopic and transgenic mouse glioma models. IDO deficiency was also associated with lower GITR expression levels on Tregs. Interestingly, the long-term survival advantage conferred by IDO deficiency was lost in T-cell-deficient mice.
Conclusions: These clinical and preclinical data confirm that IDO expression increases the recruitment of immunosuppressive Tregs that lead to tumor outgrowth. In contrast, IDO deficiency decreases Treg recruitment and enhances T-cell-mediated tumor rejection. Thus, the data suggest a critical role for IDO-mediated immunosuppression in glioma and support the continued investigation of IDO-Treg interactions in the context of brain tumors.
©2012 AACR.
Conflict of interest statement
Figures




Comment in
-
Regulatory T cells move in when gliomas say "I Do".Clin Cancer Res. 2012 Nov 15;18(22):6086-8. doi: 10.1158/1078-0432.CCR-12-2801. Epub 2012 Oct 10. Clin Cancer Res. 2012. PMID: 23052252 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl Jour Med. 2005;352:987–996. - PubMed
-
- El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105:430–437. - PubMed
-
- Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

