Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols
- PMID: 22932756
- PMCID: PMC3490606
- DOI: 10.1104/pp.112.204438
Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols
Abstract
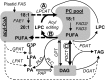
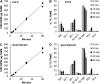
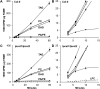
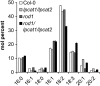
Triacylglycerols (TAG) in seeds of Arabidopsis (Arabidopsis thaliana) and many plant species contain large amounts of polyunsaturated fatty acids (PUFA). These PUFA are synthesized on the membrane lipid phosphatidylcholine (PC). However, the exact mechanisms of how fatty acids enter PC and how they are removed from PC after being modified to participate in the TAG assembly are unclear, nor are the identities of the key enzymes/genes that control these fluxes known. By reverse genetics and metabolic labeling experiments, we demonstrate that two genes encoding the lysophosphatidylcholine acyltransferases LPCAT1 and LPCAT2 in Arabidopsis control the previously identified "acyl-editing" process, the main entry of fatty acids into PC. The lpcat1/lpcat2 mutant showed increased contents of very-long-chain fatty acids and decreased PUFA in TAG and the accumulation of small amounts of lysophosphatidylcholine in developing seeds revealed by [¹⁴C]acetate-labeling experiments. We also showed that mutations in LPCATs and the PC diacylglycerol cholinephosphotransferase in the reduced oleate desaturation1 (rod1)/lpcat1/lpcat2 mutant resulted in a drastic reduction of PUFA content in seed TAG, accumulating only one-third of the wild-type level. These results indicate that PC acyl editing and phosphocholine headgroup exchange between PC and diacylglycerols control the majority of acyl fluxes through PC to provide PUFA for TAG synthesis.
Figures

References
-
- Bates PD, Browse J. (2011) The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J 68: 387–399 - PubMed
-
- Bates PD, Ohlrogge JB, Pollard M. (2007) Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem 282: 31206–31216 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

